��Ŀ����
����Ŀ�������������ʣ��� NaOH ��Һ �� Һ�� ��BaCO3 ���� �� ����KHSO4�� Fe ( OH )3���� �� ͭ �� CO2 �� CH3COOH��
��1���������������ڻ�������_____������ţ����������������ڷǵ���ʵ���_____������ţ���
��2�������ʢݽ���ͨ�磬�۲쵽����������������_____�����ϴ��������ܵ������_____������ţ���
��3��д���ٺ͢��ˮ��Һ��Ӧ�����ӷ���ʽ________��
��4��д���ܵĵ��뷽��ʽ_____��
��5���������ܵ�ˮ��Һ�м��������ۣ�������Ӧ�����ӷ���ʽΪ_____��
��6���ں�0.4mol �ٵ���Һ�л���ͨ���״����6.72LCO2�����屻ȫ�����գ���Ӧ����Һ��������_____���ѧʽ����
���𰸡� �٢� �ڢ� ������ɫ���������ɫ��dz �ܢ� ![]()
![]()
![]() Na2CO3��NaHCO3
Na2CO3��NaHCO3
��������������������⿼�����ʵķ��࣬����ʺͷǵ���ʵ��жϣ����ʵ����ԭ����ĵ�Ӿ�����뷽��ʽ�����ӷ���ʽ����д�������ʵ����йصIJ�����ж���
��1����NaOH��Һ���ڻ����Ȳ��ǵ����Ҳ���Ƿǵ���ʣ���Һ�����ڴ�������ڷǵ���ʣ���BaCO3�������ڴ�������ڵ���ʣ�������KHSO4���ڴ�������ڵ���ʣ���Fe��OH��3�������ڻ����Ȳ��ǵ����Ҳ���Ƿǵ���ʣ���ͭ���ڽ������ʣ��Ȳ��ǵ����Ҳ���Ƿǵ���ʣ���CO2���ڴ�������ڷǵ��������CH3COOH���ڴ��������������������ڻ��������٢������ڷǵ���ʵ����ڢ���
��2������Fe��OH��3�����н���������ɣ���Fe��OH��3����ͨ�磬�ɹ۲쵽������ɫ���������ɫ��dz���������ڴ���������ڢۢܢޢߢ����������ƶ����������ӵ������������ɵ��ӵ���������Щ���������ܵ�������ܢ���
��3����������ˮ��Һ��Ӧ�Ļ�ѧ����ʽΪ��NaOH+CH3COOH=CH3COONa+H2O��NaOH��CH3COONa�ij����ӣ�CH3COOH��H2O�Ի�ѧʽ���������ӷ���ʽΪCH3COOH+OH-=CH3COO-+H2O��
��4��KHSO4����ǿ�����ʽ�Σ�����ʱ�ĵ��뷽��ʽΪKHSO4=K++HSO4-��
��5��KHSO4��ˮ��Һ�еĵ��뷽��ʽΪKHSO4=K++H++SO42-��KHSO4��ˮ��Һ�����ԣ���KHSO4ˮ��Һ�м�������BaCO3����Ӧ�����ӷ���ʽΪBaCO3+2H++SO42-=BaSO4+H2O+CO2����
��6��NaOH��Һ��ͨ��CO2���ܷ����ķ�Ӧ����CO2+2NaOH=Na2CO3+H2O��CO2+NaOH=NaHCO3��1:1![]() n��NaOH����n��CO2��=0.4mol��
n��NaOH����n��CO2��=0.4mol�� ![]() =4��3
=4��3![]() 2:1�����Է�Ӧ��������Һ��������Na2CO3��NaHCO3��
2:1�����Է�Ӧ��������Һ��������Na2CO3��NaHCO3��

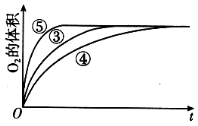
����Ŀ��ijʵ��С����H2O2�ֽ�Ϊ�����о�Ũ�ȡ���������Һ����ԶԷ�Ӧ���ʵ�Ӱ�죬�ڳ����°������·������ʵ�顣
ʵ���� | ��Ӧ�� | ���� |
�� | 10 mL 2% H2O2��Һ | �� |
�� | 10 mL 5% H2O2��Һ | �� |
�� | 10 mL 5% H2O2��Һ | 1 mL 0.1 mol L-1 FeCl3��Һ |
�� | 10 mL 5% H2O2��Һ+����HCl��Һ | 1 mL 0.1 mol L-1 FeCl3��Һ |
�� | 10 mL 5% H2O2��Һ+����NaOH��Һ | 1 mL 0.1 mol L-1 FeCl3��Һ |
��1�������ܼӿ컯ѧ��Ӧ���ʵ�ԭ����__________________________��
��2��ʵ����������Ŀ����_____________________��ʵ��ʱ���ڽϳ�ʱ��û�й۲쵽������������ó����ۡ�������ʾ��ͨ��������H2O2�ȶ������ֽ⡣Ϊ�˴ﵽʵ��Ŀ�ģ����ԭʵ�鷽���ĸĽ���_____________________��
��3��д��ʵ�����Ļ�ѧ��Ӧ����ʽ��______________��
��4��ʵ�������������У�������������������ʱ��仯�Ĺ�ϵ��ͼ��������ͼ�ܹ��ó���ʵ�������____________________________��