��Ŀ����
���ᡢ�����������������Ҫ���ᡣ
��1����ҵ����������������ʱ����Ҫ�õ�����̬��Ӧ����_______��
��2����ҵ����������������ʱ���õ�����ͬ����Ҫ�豸��_______��
��3����ҵ���������Ƶø�Ũ�ȵ����������ᣬ��ʹ�õ���ͬ������_______��
��4��Ũ������Ũ���ᱩ¶�ڿ����У���ͬ��������_______��
��5��Ũ������Ũ���ᶼ���������������������ͬԭ����_______��
��6��ʵ�����Ʊ�H2��CO2��H2S��SO2ʱ���������Ũ������Ʊ�����ͬ������_______��
��1����ҵ����������������ʱ����Ҫ�õ�����̬��Ӧ����_______��
��2����ҵ����������������ʱ���õ�����ͬ����Ҫ�豸��_______��
��3����ҵ���������Ƶø�Ũ�ȵ����������ᣬ��ʹ�õ���ͬ������_______��
��4��Ũ������Ũ���ᱩ¶�ڿ����У���ͬ��������_______��
��5��Ũ������Ũ���ᶼ���������������������ͬԭ����_______��
��6��ʵ�����Ʊ�H2��CO2��H2S��SO2ʱ���������Ũ������Ʊ�����ͬ������_______��
��1����Ҫ�õ�H2��
��������Cl2��H2���ϳ�HCl���������ð��������������Ƶ�NH3�����ϳ�NH3������H2��
��2����Ҫ�豸��������������HNO3�Ǹ�������¯��
��3��ŨH2SO4��
ŨH2SO4����ˮ����
��4������������
��5����������ŨH2SO4��ŨHNO3�ۻ���
��6��SO2��
��������Cl2��H2���ϳ�HCl���������ð��������������Ƶ�NH3�����ϳ�NH3������H2��
��2����Ҫ�豸��������������HNO3�Ǹ�������¯��
��3��ŨH2SO4��
ŨH2SO4����ˮ����
��4������������
��5����������ŨH2SO4��ŨHNO3�ۻ���
��6��SO2��
Zn��ŨH2SO4���Ȳ���SO2��CaCO3������ŨH2SO4��Ӧ���ף�H2S�ᱻŨH2SO4������

��ϰ��ϵ�д�
�����Ŀ

 �������йظ÷�Ӧ��˵����ȷ���� ( )
�������йظ÷�Ӧ��˵����ȷ���� ( )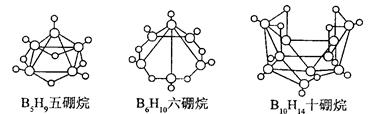
 5B2O3 ʮ9H2O ��l molB5H9��ȫȼ��ת��25mol����
5B2O3 ʮ9H2O ��l molB5H9��ȫȼ��ת��25mol����