题目内容
9.向100mL某M(OH)2澄清溶液中加入过量的NaHCO3溶液,生成了MCO3沉淀,过滤后将沉淀置于足量盐酸中,在标准状况下收集到4.48L气体.将滤液加水稀释至250mL,取出25mL恰好与20mL盐酸完全反应,在标准状况下收集到1.12L气体.(1)M(OH)2澄清溶液与NaHCO3溶液反应的离子方程式为:M2++2OH?+2HCO3-═MCO3↓+CO32-+2H2O.
(2)要计算金属M的相对原子质量,还必须提供下列哪些数据B
A.NaHCO3的物质的量浓度(设为3.5mol•L-1)
B.MCO3的质量(设为39.4g)
C.与MCO3反应的盐酸的物质的量浓度(设为1mol•L-1)
D.题设条件充足,不需要补充数据
(3)试计算
①金属M的相对原子质量
②加入的NaHCO3溶液中所含的NaHCO3的质量.
分析 (1)M(OH)2澄清溶液与过量NaHCO3溶液反应生成MCO3沉淀、碳酸钠和水,据此写出反应的离子方程式
(2)计算金属M的原子量,只需知道M的质量和物质的量即可,物质的量可以由题中MCO3与盐酸反应生成的二氧化碳的量计算;
(3)①根据关系式计算出生成沉淀的物质的量,再根据MCO3的质量(设为39.4g)及M=mnmn计算出沉淀的摩尔质量,最后得出金属M的相对原子质量;
②要计算总的NaHCO3,可以根据在反应中所有的HCO3-最终全部转化为二氧化碳气体来计算,最后生成的CO2的物质的量与原溶液中的碳酸氢钠的物质的量相等,最后根据m=nM计算出碳酸氢钠的质量.
解答 解:(1)M(OH)2澄清溶液与过量NaHCO3溶液反应生成MCO3、碳酸钠和水,反应的离子方程式为:M2++2OH?+2HCO3-═MCO3↓+CO32-+2H2O,
故答案为:M2++2OH?+2HCO3-═MCO3↓+CO32-+2H2O;
(2)计算金属M的原子量,只需知道M的质量和物质的量即可,物质的量可以由题中MCO3与盐酸的反应中计算出,因此只需中知道B项:M的碳酸盐的质量即可,
故答案为:B;
(3)①设MCO3的物质的量为x,则
MCO3 ~CO2,
1mol 22.4L
x 4.48L
则:x=1mol×4.48L22.4L=0.2mol,
根据B选项中给出MCO3质量为39.4g,则MCO3的摩尔质量为:39.4g0.2mol=197g/mol,则MCO3的相对分子质量为197,
所以M的原子量为:197-60=137,
答:金属M的相对原子质量为197;
②反应中所有的HCO3-最终全部转化为二氧化碳气体,即:最后生成的CO2的物质的量与原溶液中的碳酸氢钠的物质的量相等,
根据题意,生成总的二氧化碳的体积为:4.48L+1.12L×250mL25mL=15.68L,其物质的量为:15.68L22.4L/mol=0.7mol,
根据碳原子守恒,则原碳酸氢钠溶液中含有碳酸氢钠的物质的量为0.7mol,质量为:84g/mol×0.7mol=58.8g,
答:加入的NaHCO3溶液中所含的NaHCO3的质量为58.8g.
点评 本题考查了数据缺省型的化学计算,题目难度中等,明确发生反应实质为解答关键,注意掌握质量守恒定律在化学计算中的应用方法,试题培养了学生的分析能力及化学计算能力.

| A. | Cl一的结构示意图: | B. | CH4分子的球棍模型: | ||
| C. | 氯化镁的电子式: | D. | 8个中子的碳原子的核素符号:12C |
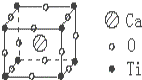 已知某化合物是由钙、钛、氧三种元素组成的晶体,其晶胞结构如图所示,则该物质的化学式为( )
已知某化合物是由钙、钛、氧三种元素组成的晶体,其晶胞结构如图所示,则该物质的化学式为( )| A. | CaTiO3 | B. | CaTiO6 | C. | Ca4TiO3 | D. | CaTiO12 |
| A. | 生命体中糖类与氧气的反应、生产和生活中燃料的燃烧等都是反应热效应的重要应用 | |
| B. | 能源是可以提供能量的自然资源,包括化石燃料、阳光、风力、流水、潮汐等 | |
| C. | 一个化学反应是吸收能量还是放出能量,取决于反应物总能量和生成物总能量的相对大小 | |
| D. | 在化学反应过程中,只要反应物和生成物具有相同温度,反应所吸收或放出的热量就称为化学反应的焓变 |
| A. | 水中 | B. | 乙酸中 | C. | 煤油中 | D. | 乙醇中 |
| A. | 离子键就是阴阳离子间强烈的静电作用 | |
| B. | H和Cl形成的分子中存在的是极性共价键 | |
| C. | 氯化铵中没有金属元素,所以不是离子化合物 | |
| D. | 11号与9号元素能够形成离子化合物,该化合物中存在离子键 |
| A. | 原子半径:W>Z>Y>X>M | |
| B. | XZ2、X2M4均为直线形的共价化合物 | |
| C. | W2Z2是既含离子键又含共价键的离子化合物 | |
| D. | 由X、Y、Z、M四种元素形成的化合物一定既有离子键、又有共价键 |
| A. | 8 | B. | 10 | C. | 16 | D. | 24 |
