��Ŀ����
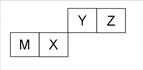
����Ԫ��X��Y��Z��W��ԭ���������������������Ϣ���±���
��1��Yλ��Ԫ�����ڱ��� ���� �壬���̬ԭ��δ�ɶԵ����� ����
��2��X�ĵ縺�Ա�W�� �����С������Y�������̬�⻯���X�������̬�⻯����Һ��������Ҫԭ���� ��
��3��Z��ͬ�����������ڵ�����Ԫ�ص�ԭ����Ƚϣ����ߵ�һ�������ɴ�С��˳��Ϊ ����Ԫ�ط��ű�ʾ����Y��Z�γɵĻ�����Ϊ ���壬��������ˮǿ��ˮ��Ļ�ѧ����ʽΪ ��
��4����һ���¶��£���һ���ݻ�������ܱ������г���1molY2��3mol������������Ӧ��Y2(g)+3H2(g) 2YH3(g) ��H=-akJ/mol���ڸ������´ﵽƽ��ʱ�ų�������ΪbkJ����ƽ�ⳣ������ʽK= ������ʼ��������г���2molYH3������ͬ�¶��´ﵽƽ��ʱ����Ӧ���������յ�����ΪckJ����a��b��c����֮��Ĺ�ϵΪ ����һ��ʽ�ӱ�ʾ����
| Ԫ�� | �����Ϣ |
| X | X�Ļ�̬ԭ�Ӻ���ֻ�������ܼ����Ҹ��ܼ���������� |
| Y | Y�Ļ�̬ԭ�����������������ڲ����������2.5�� |
| Z | Z�Ļ�̬�۵��ӽṹΪnsn-1 |
| W | W���ʳ��ڻ�ɽ�ڸ��������֣���������������������Ҫԭ��֮һ |
��1��Yλ��Ԫ�����ڱ��� ���� �壬���̬ԭ��δ�ɶԵ����� ����
��2��X�ĵ縺�Ա�W�� �����С������Y�������̬�⻯���X�������̬�⻯����Һ��������Ҫԭ���� ��
��3��Z��ͬ�����������ڵ�����Ԫ�ص�ԭ����Ƚϣ����ߵ�һ�������ɴ�С��˳��Ϊ ����Ԫ�ط��ű�ʾ����Y��Z�γɵĻ�����Ϊ ���壬��������ˮǿ��ˮ��Ļ�ѧ����ʽΪ ��
��4����һ���¶��£���һ���ݻ�������ܱ������г���1molY2��3mol������������Ӧ��Y2(g)+3H2(g) 2YH3(g) ��H=-akJ/mol���ڸ������´ﵽƽ��ʱ�ų�������ΪbkJ����ƽ�ⳣ������ʽK= ������ʼ��������г���2molYH3������ͬ�¶��´ﵽƽ��ʱ����Ӧ���������յ�����ΪckJ����a��b��c����֮��Ĺ�ϵΪ ����һ��ʽ�ӱ�ʾ����
��1��2��VA��3 ����1�֣���3�֣�
��2��С��������֮������γ���� ����1�֣�2�֣�
��3��Mg��Al��Na�����ӣ�Mg3N2+6H2O=3Mg(OH)2+2NH3�� ����2�֣�6�֣�
��4��c2(NH3)/[c(N2)c3(H2)] a=b+c ����2�֣�4�֣�
��2��С��������֮������γ���� ����1�֣�2�֣�
��3��Mg��Al��Na�����ӣ�Mg3N2+6H2O=3Mg(OH)2+2NH3�� ����2�֣�6�֣�
��4��c2(NH3)/[c(N2)c3(H2)] a=b+c ����2�֣�4�֣�
�����������������֪X��Y��Z��W�ֱ���C��N��Mg��S��
Nλ��Ԫ�����ڱ��ĵڶ����ڵ�VA�壬���̬ԭ�Ӻ�������Ų�ʽΪ1S22S2P3������3��δ�ɶԵ��ӣ�
C�ĵ縺�Ա�S��ҪС��NH3��CH4��Һ������Ϊ�����Ӽ���������
����Ԫ��������֪ͬһ����Ԫ�ش����ҵ�һ������������þ���������3S2����ȫ�����ṹ�����ȶ�����һ�����ܽϺ������Ҫ��Mg3N2�����Ӿ��壬��ˮ��Ӧ�ķ���ʽ��Mg3N2+6H2O=3Mg(OH)2+2NH3����
��ҵ�ϳɰ���ƽ�ⳣ������ʽ��K= c2(NH3)/[c(N2)c3(H2)]�����ݿ��淴Ӧ���ص�֪a��b��c����֮��Ĺ�ϵ��a=b+c��

��ϰ��ϵ�д�
 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�
�����Ŀ

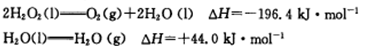
 ��˵�����������
��˵�����������
 2CA3��H��0����Ӧ10min�ﵽƽ�⣬����0.2molCA3��
2CA3��H��0����Ӧ10min�ﵽƽ�⣬����0.2molCA3��