��Ŀ����
����Ŀ����ˮ�Ǿ����Դ���⣬�Ӻ�ˮ����ȡʳ�κ���Ĺ������£�
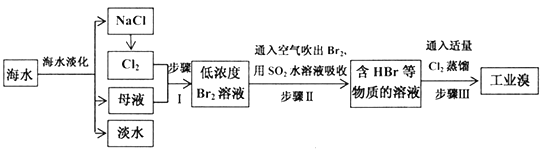
��1��д������I�����ɵ�Ũ��Br2�����ӷ���ʽ______��
��2������I���ѻ��Br2������I���ֽ�Br2��ԭΪBr-����Ŀ��Ϊ������Ԫ�أ���д������II�Ļ�ѧ����ʽ_______��
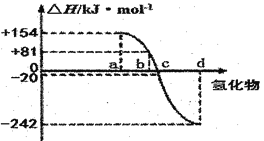
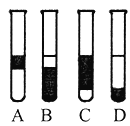
��3����3mL��ˮ�м���1mL���Ȼ�̼�������ú۲쵽�Թ���ķֲ�����Ϊ��ͼ�е�_______��
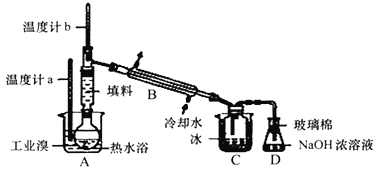
��4��ij��ѧ�о���ѧϰС��Ϊ�˽�ӹ�ҵ�����ᴿ��ķ������������й����ϣ�Br2�ķе�Ϊ59�棬����ˮ���ж��Ժ�ǿ��ʴ�ԣ������������ͼװ�ü�ͼ���������������ۣ�
��C��Һ�������ɫΪ_____��
�������ӷ���ʽ����NaOHŨ��Һ������______��
���𰸡�Cl2+2Br-=2Cl-+Br2 Br2+SO2+2H2O=2HBr+H2SO4 D �����ɫ Br2+2OH-=Br-+BrO- +H2O
��������
��ˮ�����õ��Ȼ��ƣ�����Ȼ�����Һ������״̬�Ȼ��ƻ���������������ͨ��ĸҺ�з�����Ӧ�õ���Ũ�ȵ��嵥����Һ��ͨ���ȿ����������ö�������ˮ��Һ���յõ���HBr���������Һ��ͨ���������������õ��嵥�ʣ�������Ԫ�أ�����õ���ҵ�壻
(1)����I�����ɵ�Ũ��Br2���漰�����������ӵ��û���Ӧ��
(2)���������ֽ�Br2��ԭΪBr-�������������巢��������ԭ��Ӧ��
(3)���Ȼ�̼���ܶȱ�ˮ���������������Ȼ�̼��
(4)��ҵ�������ᴿ��ķ�������Ҫ��������������Ϊ����и�����ϢBr2�ķе���59�����ᴿ������ռ�59��ʱ����֣�C��Һ��Ϊ���������Ĵ��壬����ɫΪ���غ�ɫ���������ж�����Ҫ�ü�Һ�����ա�
��1�������ܹ����������ӵõ������Ӻ͵����壬���ӷ���ʽΪ��Cl2+2Br-=2Cl-+Br2��
�ʴ��ǣ�Cl2+2Br-=2Cl-+Br2��
��2������������л�ԭ�ԣ�����������ԣ����߷���������ԭ��Ӧ��������������ᣬ����II�Ļ�ѧ����ʽΪ��Br2+SO2+2H2O=2HBr+H2SO4��
�ʴ��ǣ�Br2+SO2+2H2O=2HBr+H2SO4��
��3�����Ȼ�̼�ܹ���ȡ��ˮ�е��壬�������Ȼ�̼���ܶȴ���ˮ���ܶȣ������������ǣ���Һ�ֲ㣬���ܽ����Ȼ�̼�гʳȺ�ɫ�������²�ʳȺ�ɫ���ϲ�Ϊˮ�㣬����ɫ����D��ȷ��
�ʴ�ѡD��
��4����C��Բ����ƿ�в���Һ��Ϊ�壬��ɫΪ�����ɫ��
����:�����ɫ��
��Br2�ж��������ŷŵ������У�D��ŨNaOH��Һ�����������ջӷ��������壬��Ӧ�����ӷ���ʽΪBr2+2OH-=Br-+BrO- +H2O��
�ʴ���: Br2+2OH-=Br-+BrO- +H2O��

 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�����Ŀ�������������ʵ���Ҫ���������ʽṹ����ش��������⣺
(1)��֪Ԫ��M���������Ca5(PO4)3F��һ��Ԫ�ء�Ԫ��M����̬ԭ�����ʧȥ��1������5��������������(�������ܣ��÷���I1��I5��ʾ)�����ʾ��
I1 | I2 | I3 | I4 | I5 | |
������ | 589.8 | 1145.4 | 4912.4 | 6491 | 8153 |
Ԫ��M����̬�������ϼ���_________�ۣ����̬ԭ�ӵ����Ų�ʽΪ_________��
(2)Ca3(PO4)3F�зǽ���Ԫ�ص縺���ɴ�С��˳��Ϊ_________��
(3)PO43-������ԭ�ӵ��ӻ���ʽΪ_________�������ӵĿռ乹��Ϊ_________������Ϊ________����ȵ�������_________ (��д������)��
(4)CaF2�����ṹ��ͼ��ʾ����CaF2��������Ca2+����ҵȾ����Ca2+��ĿΪ_________����֪Ca2+��F�뾶�ֱ�Ϊa cm��b cm�������ӵ�����ΪNA��MΪĦ�������������ܶ�Ϊ________g��cm3(���ػ���)��
(5)��֪MgO��CaO�ľ���ṹ���ƣ���Ħ��Ӳ�ȵĴ�С��ϵΪ_________��ԭ��Ϊ___________��