��Ŀ����
����Ŀ����.�ϳɰ���ҵ�У�ÿ����![]() ���ų�
���ų�![]() ������
������
��1��д���ϳɰ���Ӧ���Ȼ�ѧ����ʽ___________��
��2����֪��

![]() ���������յ���������_______
���������յ���������_______![]() ��������ȡ��������
��������ȡ��������
��.�˹�������ü�ӵ绯ѧ������ȥ��л�����е�����[��ѧʽΪ![]() ]��ԭ����ͼ��
]��ԭ����ͼ��
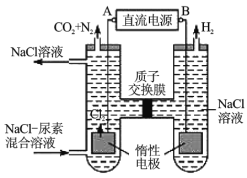
��1����Դ�ĸ���Ϊ__________������A������B������
��2���������з����ķ�Ӧ����Ϊ_________��_________��
��3������������������Һ��![]() ����ǰ��Ƚ�__________������������������С����������������
����ǰ��Ƚ�__________������������������С����������������
���𰸡�N2(g)+3H2(g)2NH3(g)��H=-92.4kJmol-1 391 B 6Cl--6e-�T3Cl2�� CO(NH2)2+3Cl2+H2O�TN2+CO2+6HCl ����
��������
��(1)���ݺϳɰ��Ļ�ѧ����ʽ������H��������д�Ȼ�ѧ����ʽ��
(2)�����ʱ���H=��Ӧ����ܼ���-��������ܼ��ܼ��㣻
���ݵ��������ҵ缫����ΪH2��������ӦΪ��6H2O+6e-�T6OH-+3H2��(��6H++6e-�T3H2��)��������ӦΪ��6Cl--6e-�T3Cl2����Cl2����CO(NH2)2����N2��CO2���ݴ˷������
��(1)�ϳɰ��Ļ�ѧ����ʽΪN2(g)+3H2(g)2NH3(g)��ÿ����2molNH3���ų�92.4kJ���������Ժϳɰ���Ӧ���Ȼ�ѧ����ʽΪN2(g)+3H2(g)2NH3(g)��H=-92.4kJmol-1���ʴ�Ϊ��N2(g)+3H2(g)2NH3(g)��H=-92.4kJmol-1��
(2)��N-H���ļ���Ϊx��N2(g)+3H2(g)2NH3(g)��H=-92.4kJmol-1����945.8kJ/mol+3��436kJ/mol-6x=-92.4kJ/mol�����x=391kJ/mol����1moIN-H���������յ���������391kJ���ʴ�Ϊ��391��
��(1)��ͼ��֪�����ҵ缫����ΪH2������Һ�е������ӵõ��˵��ӣ������˻�ԭ��Ӧ�����ҵ缫Ϊ����������BΪ��Դ�ĸ�����AΪ��Դ���������ʴ�Ϊ��B��
(2)����ʧ���ӣ�����������Ӧ������������ӦΪ6Cl--6e-�T3Cl2�������ɵ�Cl2����CO(NH2)2����N2��CO2����ѧ����ʽΪCO(NH2)2+3Cl2+H2O�TN2+CO2+6HCl���ʴ�Ϊ��6Cl--6e-�T3Cl2����CO(NH2)2+3Cl2+H2O�TN2+CO2+6HCl��
(3)������ӦΪ6H2O+6e-�T6OH-+3H2��(��6H++6e-�T3H2��)��������ӦΪ6Cl--6e-�T3Cl2�������ɵ�Cl2����CO(NH2)2�ķ�ӦΪCO(NH2)2+3Cl2+H2O�TN2+CO2+6HCl������������Ӧʽ�͵����غ��֪�������������ϲ�����OH-��H+����Ŀ��ȣ��������з�Ӧ������H+ͨ�����ӽ���Ĥ������������OH-ǡ�÷�Ӧ����ˮ�������������е��ǰ����Һ��pH���䣬�ʴ�Ϊ�����䡣

����Ŀ��ij��θҩ��ֹ���Ϊ̼��ƣ��ⶨÿƬ��̼��ƺ����ķ��������¼�����������ҩƬ�е������ɷֲ������ᷴӦ��Ҳ�����������Ʒ�Ӧ����ʵ�鲽�����£�
������![]() ϡ�����
ϡ�����![]() ��Һ��
��Һ��![]()
��ȡһ��ҩƬ��![]() ����������
����������![]() ����ˮ
����ˮ
�ۼ���![]() ϡ����
ϡ����
����![]() ��Һ�к������ᣬ��ȥ���Ϊ
��Һ�к������ᣬ��ȥ���Ϊ![]() ��
��
��ش��������⣺
��1���ⶨ�����з�����Ӧ�����ӷ���ʽ_________��
��2����������![]() ϡ�������ò�����������Ͳ���ձ���________��
ϡ�������ò�����������Ͳ���ձ���________��
��3�����ѡ�÷�̪��ָʾ�����ζ��ﵽ�յ������Ϊ____��
��4��ijͬѧ�Ĵβⶨ��![]() �������£�
�������£�
�ⶨ���� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| 13.40 | 11.90 | 12.10 | 12.00 |
�������λͬѧ��ʵ�����ݣ�����ҩƬ��̼��Ƶ���������Ϊ_____��
��5������ʵ������д������в�������ʹ����̼��Ƶ���������ƫ�ߵ���_________��
a ��û����ϴ�ļ�ʽ�ζ���װ![]() ��Һ���еζ�
��Һ���еζ�
b ��û����ϴ����ʽ�ζ�����ȡ![]() ϡ�����ܽ���Ʒ
ϡ�����ܽ���Ʒ
c ��![]() ��Һ�ζ�ʱ����ʼ����ƽ�ӣ��յ㸩��
��Һ�ζ�ʱ����ʼ����ƽ�ӣ��յ㸩��
d װ![]() ��Һ�ĵζ��ܣ��ζ�ǰ���������ݣ��ζ������������ݡ�
��Һ�ĵζ��ܣ��ζ�ǰ���������ݣ��ζ������������ݡ�
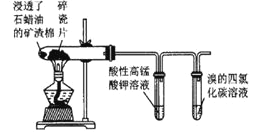
����Ŀ������ʵ��װ���������������Լ�������ͽ��۾���ȷ����
ѡ�� | װ�� | ���� | ���� |
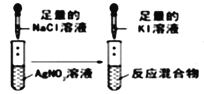
A |
| װ�â�����dz��ɫ�������� | ����Ũ��ˮ����ȡ����Ӧ |
B |
| ���������Һ��ɫ��������Ȼ�̼��Һ��ɫ | ʯ���͵ķֽ�����к�����ϩ |
C |
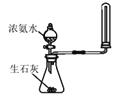
| ��Һ©��Һ�����£���ƿ�в����������� | �����Ʊ����ռ�һ�����İ��� |
D |
| �Թ������γ��ְ�ɫ����ɫ���� | ˵�� Ksp(AgCl)>Ksp(AgI) |
A.AB.BC.CD.D