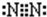��Ŀ����
A��B��C��D��EΪǰ��������ԭ���������������Ԫ�أ���ص���Ϣ���£�| Ԫ�� | �����Ϣ |
| A | AԪ��ԭ�Ӻ���ֻ�������ܼ�����ÿ���ܼ��Ϻ�����ȵĵ����� |
| B | �ǿ����к�����ḻ��Ԫ�� |
| C | ������Ԫ���У�C�Ľ�������ǿ |
| D | ��̬ԭ�ӵ����ܲ�����7���˶�״̬��ͬ�ĵ��� |
| E | һ�ֺ��ص�������Ϊ63��������Ϊ34 |
��1��A������γ�һ�ַ���ʽΪA2H4�Ļ�����÷����д��ڦҼ��ͦм���Ŀ��Ϊ______��
��2��H-A��H-B���ֹ��ۼ��У����ܽϴ����______��H-A��H-D���ֹ��ۼ��У����ļ��Խ�ǿ����______��
��3��Eλ�����ڱ��е�λ����______������E��B������������ˮ�����ϡ��Һ��Ӧ�����ӷ���ʽΪ______��
��4���������������仯ʾ��ͼ1����д��BO��AO2��Ӧ���Ȼ�ѧ����ʽ______��
��5��C������������Ӧ��ˮ����ΪM��M�к��еĻ�ѧ������Ϊ______����һ������D2ͨ��һ��Ũ��M��ˮ��Һ�У�����ǡ����ȫ��Ӧʱ���������������ֺ�DԪ�ص����ӣ������������ӵ����ʵ�����n���뷴Ӧʱ�䣨t���ı仯ʾ��ͼ��ͼ2��ʾ����д��t2ʱ���ܷ�Ӧ�Ļ�ѧ����ʽ
���𰸡�������A��B��C��D��EΪǰ��������ԭ���������������Ԫ�أ�AԪ��ԭ�Ӻ���ֻ�������ܼ�����ÿ���ܼ��Ϻ�����ȵĵ����������������Ų�Ϊ1s22s22p2����AΪ̼Ԫ�أ�B�ǿ����к�����ḻ��Ԫ�أ���BΪ��Ԫ�أ�������Ԫ���У�C�Ľ�������ǿ����CΪNaԪ�أ�D��̬ԭ�ӵ����ܲ�����7���˶�״̬��ͬ�ĵ��ӣ����������Ų�Ϊ1s22s22p23s23p5����DΪClԪ�أ�E��һ�ֺ��ص�������Ϊ63��������Ϊ34����E��������Ϊ63-34=29��ΪCuԪ�أ��ݴ˽��
����⣺A��B��C��D��EΪǰ��������ԭ���������������Ԫ�أ�AԪ��ԭ�Ӻ���ֻ�������ܼ�����ÿ���ܼ��Ϻ�����ȵĵ����������������Ų�Ϊ1s22s22p2����AΪ̼Ԫ�أ�B�ǿ����к�����ḻ��Ԫ�أ���BΪ��Ԫ�أ�������Ԫ���У�C�Ľ�������ǿ����CΪNaԪ�أ�D��̬ԭ�ӵ����ܲ�����7���˶�״̬��ͬ�ĵ��ӣ����������Ų�Ϊ1s22s22p23s23p5����DΪClԪ�أ�E��һ�ֺ��ص�������Ϊ63��������Ϊ34����E��������Ϊ63-34=29��ΪCuԪ�أ�
��1��A������γ�һ�ַ���ʽΪC2H4�������д���1��C=C��4��C-H������Ϊ�Ҽ���˫������1�Ҽ����м���������ϩ�����к���5���Ҽ���1���м��������ЦҼ��ͦм���Ŀ��Ϊ5��1���ʴ�Ϊ��5��1��
��2��̼����ĵ縺�����С��C-H�����ڷǼ��Թ��ۼ���������ĵ縺�����ܴ����Ե�����ǿ�ҵ�ƫ���ڵ�ԭ�ӣ�ʹ�ü��ļ��Ժܴ������ѣ�H-C�ļ��ܸ���H-N���ǽ�����Cl��C����H-Cl���ļ��Ա�H-Cǿ��
�ʴ�Ϊ��H-C��H-Cl��
��3��E��������Ϊ63-34=29��ΪCuԪ�أ�λ�����ڱ��е������ڵ�IB�壬����Cu��ϡ���ᷴӦ�����ӷ���ʽΪ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
�ʴ�Ϊ���������ڵ�IB�壻3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
��4�����������仯ʾ��ͼ1��֪��1molNO��1molCO2��Ӧ����1molNO2��1molCO���յ�����Ϊ234kJ���ʸ÷�Ӧ���Ȼ�ѧ����ʽΪ��NO��g��+CO2 ��g��=NO2 ��g��+CO ��g����H=+234 kJ/mol��
�ʴ�Ϊ��NO��g��+CO2 ��g��=NO2 ��g��+CO ��g����H=+234 kJ/mol��
��5��C������������Ӧ��ˮ����ΪNaOH���������ӻ������������������ԭ������ԭ��֮���γɼ��Լ���NaOH�к������Ӽ������Լ���
��һ������Cl2ͨ��һ��Ũ��NaOH��ˮ��Һ�У�����ǡ����ȫ��Ӧʱ���������������ֺ�ClԪ�ص����ӣ���ͼ��֪t2ʱ������ClO-��ClO3-���������ʵ���֮��Ϊ2��1�����ݵ���ת���غ��֪������Cl-��t2ʱ���ܷ�Ӧ�Ļ�ѧ����ʽΪ��5Cl2+10NaOH=7NaCl+2NaClO+NaClO3+5H2O��
�ʴ�Ϊ�����Ӽ������Լ���5Cl2+10NaOH=7NaCl+2NaClO+NaClO3+5H2O��
���������⿼��ṹ����λ�ù�ϵ���ۺ�Ӧ�á���ѧ�����Ȼ�ѧ����ʽ�����û�ѧ����ȣ���Ŀ��Ϊ�ۺϣ��Ѷ��еȣ���2���м��ܱȽ����״��㡢�ѵ㣬ע�����ռ�������������ϵ�������ԣ�
����⣺A��B��C��D��EΪǰ��������ԭ���������������Ԫ�أ�AԪ��ԭ�Ӻ���ֻ�������ܼ�����ÿ���ܼ��Ϻ�����ȵĵ����������������Ų�Ϊ1s22s22p2����AΪ̼Ԫ�أ�B�ǿ����к�����ḻ��Ԫ�أ���BΪ��Ԫ�أ�������Ԫ���У�C�Ľ�������ǿ����CΪNaԪ�أ�D��̬ԭ�ӵ����ܲ�����7���˶�״̬��ͬ�ĵ��ӣ����������Ų�Ϊ1s22s22p23s23p5����DΪClԪ�أ�E��һ�ֺ��ص�������Ϊ63��������Ϊ34����E��������Ϊ63-34=29��ΪCuԪ�أ�
��1��A������γ�һ�ַ���ʽΪC2H4�������д���1��C=C��4��C-H������Ϊ�Ҽ���˫������1�Ҽ����м���������ϩ�����к���5���Ҽ���1���м��������ЦҼ��ͦм���Ŀ��Ϊ5��1���ʴ�Ϊ��5��1��
��2��̼����ĵ縺�����С��C-H�����ڷǼ��Թ��ۼ���������ĵ縺�����ܴ����Ե�����ǿ�ҵ�ƫ���ڵ�ԭ�ӣ�ʹ�ü��ļ��Ժܴ������ѣ�H-C�ļ��ܸ���H-N���ǽ�����Cl��C����H-Cl���ļ��Ա�H-Cǿ��
�ʴ�Ϊ��H-C��H-Cl��
��3��E��������Ϊ63-34=29��ΪCuԪ�أ�λ�����ڱ��е������ڵ�IB�壬����Cu��ϡ���ᷴӦ�����ӷ���ʽΪ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
�ʴ�Ϊ���������ڵ�IB�壻3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
��4�����������仯ʾ��ͼ1��֪��1molNO��1molCO2��Ӧ����1molNO2��1molCO���յ�����Ϊ234kJ���ʸ÷�Ӧ���Ȼ�ѧ����ʽΪ��NO��g��+CO2 ��g��=NO2 ��g��+CO ��g����H=+234 kJ/mol��
�ʴ�Ϊ��NO��g��+CO2 ��g��=NO2 ��g��+CO ��g����H=+234 kJ/mol��
��5��C������������Ӧ��ˮ����ΪNaOH���������ӻ������������������ԭ������ԭ��֮���γɼ��Լ���NaOH�к������Ӽ������Լ���
��һ������Cl2ͨ��һ��Ũ��NaOH��ˮ��Һ�У�����ǡ����ȫ��Ӧʱ���������������ֺ�ClԪ�ص����ӣ���ͼ��֪t2ʱ������ClO-��ClO3-���������ʵ���֮��Ϊ2��1�����ݵ���ת���غ��֪������Cl-��t2ʱ���ܷ�Ӧ�Ļ�ѧ����ʽΪ��5Cl2+10NaOH=7NaCl+2NaClO+NaClO3+5H2O��
�ʴ�Ϊ�����Ӽ������Լ���5Cl2+10NaOH=7NaCl+2NaClO+NaClO3+5H2O��
���������⿼��ṹ����λ�ù�ϵ���ۺ�Ӧ�á���ѧ�����Ȼ�ѧ����ʽ�����û�ѧ����ȣ���Ŀ��Ϊ�ۺϣ��Ѷ��еȣ���2���м��ܱȽ����״��㡢�ѵ㣬ע�����ռ�������������ϵ�������ԣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
����ѧ--ѡ��3�����ʽṹ�����ʡ�
��������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣮
��1��Gλ�� �� �����۵����Ų�ʽΪ ��
��2��B��̬ԭ����������ߵĵ��ӣ���������ڿռ��� ������ԭ�ӹ����
�Σ�
��3������Cԭ�ӵĵ����Ų�ͼ ��
��4����֪BA5Ϊ���ӻ����д�������ʽ ��
��5��DE3����ԭ�ӵ��ӻ���ʽΪ ���ü۲���ӶԻ��������Ʋ���ռ乹��Ϊ ��
��6���õ���ʽ��ʾFԪ����EԪ���γɻ�������γɹ��� ��
��������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣮
| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ� I1=738kJ/mol I2=1451kJ/mol I3=7733kJ/mol I4=10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص������������������IJ�Ϊ4 |
| F��ǰ�������е縺����С��Ԫ�� |
| G�����ڱ��ĵ����� |
��2��B��̬ԭ����������ߵĵ��ӣ���������ڿռ���
�Σ�
��3������Cԭ�ӵĵ����Ų�ͼ
��4����֪BA5Ϊ���ӻ����д�������ʽ
��5��DE3����ԭ�ӵ��ӻ���ʽΪ
��6���õ���ʽ��ʾFԪ����EԪ���γɻ�������γɹ���
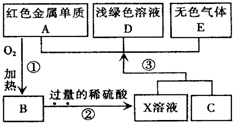 A��B��C��D��EΪ��ѧ��ѧ�������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�
A��B��C��D��EΪ��ѧ��ѧ�������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�