��Ŀ����
��ҵ�Ϻϳɰ�����һ�������½������·�Ӧ��
N2��g��+3H2��g��?2NH3��g������H=-92.44kJ/mol���䲿�ֹ����������£�
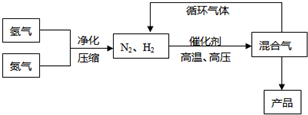
��Ӧ��ϵ�и���ֵIJ������ʼ��±���
�ش��������⣺
��1��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��K=______�������¶ȵ����ߣ�Kֵ______��������С�����䣩��
��2��ƽ�ⳣ��KֵԽ����______������ţ���
A��N2��ת����Խ�� B��NH3�IJ���Խ��
C��ԭ����N2�ĺ���Խ�� D����ѧ��Ӧ����Խ��
��3���ϳɰ���Ӧ��ƽ�ⳣ����С�������ڹ�ҵ�ϲ�ȡ����ѭ�������̣�����Ӧ��ͨ���ѻ��������¶Ƚ��͵�______ʹ______�������������ѭ����������______��
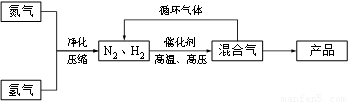
N2��g��+3H2��g��?2NH3��g������H=-92.44kJ/mol���䲿�ֹ����������£�

��Ӧ��ϵ�и���ֵIJ������ʼ��±���
| ���� | ���� | ���� | �� |
| �۵㣨�棩 | -210.01 | -252.77 | -77.74 |
| �е㣨�棩 | -195.79 | -259.23 | -33.42 |
��1��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��K=______�������¶ȵ����ߣ�Kֵ______��������С�����䣩��
��2��ƽ�ⳣ��KֵԽ����______������ţ���
A��N2��ת����Խ�� B��NH3�IJ���Խ��
C��ԭ����N2�ĺ���Խ�� D����ѧ��Ӧ����Խ��
��3���ϳɰ���Ӧ��ƽ�ⳣ����С�������ڹ�ҵ�ϲ�ȡ����ѭ�������̣�����Ӧ��ͨ���ѻ��������¶Ƚ��͵�______ʹ______�������������ѭ����������______��
��1��ƽ�ⳣ��K=
=
�����ݷ�ӦN2��g��+3H2��g��?2NH3��g������H=-92.44kJ/mol����֪��Ӧ�Ƿ��ȷ�Ӧ�������¶ȵ����ߣ�K��С���ʴ�Ϊ��
������
��2��ƽ�ⳣ��KֵԽ��˵����ѧ��Ӧ���ҽ��еij��ף���Ӧ���ת����Խ�ߣ�������NH3�IJ���Խ��ѡAB��
��3�����������֪�����ķе���-33.42�����Խ��¶Ƚ��͵�-33.42���ð�����������������͵���������ѭ�����ã��ʴ�Ϊ��-33.42��NH3��N2��H2��
| ������ƽ��Ũ��ϵ���η��ij˻� |
| ��Ӧ��ƽ��Ũ��ϵ���η��ij˻� |
| [NH3]2 |
| [N2][H2]3 |
| [NH3]2 |
| [N2][H2]3 |
��2��ƽ�ⳣ��KֵԽ��˵����ѧ��Ӧ���ҽ��еij��ף���Ӧ���ת����Խ�ߣ�������NH3�IJ���Խ��ѡAB��
��3�����������֪�����ķе���-33.42�����Խ��¶Ƚ��͵�-33.42���ð�����������������͵���������ѭ�����ã��ʴ�Ϊ��-33.42��NH3��N2��H2��

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ



 2NO(g) ��H
2NO(g) ��H ��������ͬ���������ʱ����Һ�����ԣ���������pH__________
��������ͬ���������ʱ����Һ�����ԣ���������pH__________ ������ڡ�����С�ڡ����ڡ�����
������ڡ�����С�ڡ����ڡ����� 2NH3��g���ش��������⣺
2NH3��g���ش��������⣺ ����c��
����c�� ����c��H2SO3��]��c��
����c��H2SO3��]��c�� ����c��NH3��H2O��
����c��NH3��H2O��