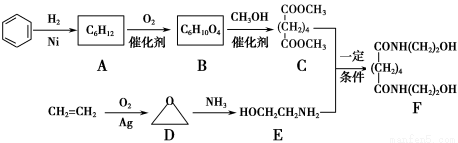
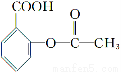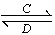ЬтФПФкШн
ЙЄвЕЩЯвдЬМЫсУЬПѓЮЊжївЊдСЯЩњВњMnO2ЕФЙЄвеСїГЬШчЯТЃК
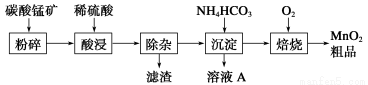
гаЙиЧтбѕЛЏЮяПЊЪМГСЕэКЭГСЕэЭъШЋЕФpHШчЯТБэЃК
ЧтбѕЛЏЮя | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 | Pb(OH)2 | Mn(OH)2 |
ПЊЪМГСЕэЕФpH | 3.3 | 1.5 | 6.5 | 4.2 | 8.0 | 8.3 |
ГСЕэЭъШЋЕФpH | 5.2 | 3.7 | 9.7 | 6.7 | 8.8 | 9.8 |
ЧыЛиД№ЯТСаЮЪЬтЃК
(1)ЫсНўЧАНЋЬМЫсУЬПѓЗлЫщЕФзїгУЪЧ__________________ЁЃ
(2)ЫсНўКѓЕФШмвКжаКЌгаMn2ЃЋЁЂSO42ЁЊЃЌСэКЌгаЩйСПFe2ЃЋЁЂFe3ЃЋЁЂAl3ЃЋЁЂCu2ЃЋЁЂPb2ЃЋЕШЃЌЦфГ§дгЙ§ГЬШчЯТЃК
ЂйМгШыMnO2НЋFe2ЃЋбѕЛЏЃЌЦфРызгЗДгІЗНГЬЪНЮЊ__________________________ЁЃ
ЂкМгШыCaOНЋШмвКЕФpHЕїЕН5.2ЁЋ6.0ЃЌЦфжївЊФПЕФЪЧ
_____________________________________________________________ЁЃ
ЂлМгШыBaSЃЌГ§ШЅCu2ЃЋЁЂPb2ЃЋКѓЃЌдйМгШыNaFШмвКЃЌГ§ШЅ______________________ЁЃ
(3)ДгШмвКAжаЛиЪеЕФжївЊЮяжЪЪЧ________________ЃЌИУЮяжЪГЃгУзїЛЏЗЪЁЃ
(4)MnO2ДжЦЗжаКЌгаЩйСПMn3O4ЃЌПЩвдгУЯЁСђЫсДІРэЃЌНЋЦфзЊЛЏЮЊMnSO4КЭMnO2ЃЌШЛКѓдйгУбѕЛЏМСНЋMn2ЃЋзЊЛЏЮЊMnO2ЃЌжЦЕУгХжЪMnO2ЁЃаДГіMn3O4гыЯЁСђЫсЗДгІЕФЛЏбЇЗНГЬЪНЃК______________________________ЁЃ
(1)діДѓНгДЅУцЛ§ЃЌМгПьЗДгІЫйТЪ(ЪЙЗДгІИќГфЗж)
(2)Ђй2Fe2ЃЋЃЋMnO2ЃЋ4HЃЋ=2Fe3ЃЋЃЋMn2ЃЋЃЋ2H2O
ЂкГ§ШЅFe3ЃЋЁЂAl3ЃЋЁЁЂлCa2ЃЋ
(3)(NH4)2SO4
(4)Mn3O4ЃЋ2H2SO4=2MnSO4ЃЋMnO2ЃЋ2H2O
ЁОНтЮіЁП(1)НЋЬМЫсУЬПѓЗлЫщЃЌПЩвддіДѓгыH2SO4ЕФНгДЅУцЛ§ЃЌМгПьЛЏбЇЗДгІЫйТЪЁЃ(2)ИљОнГСЕэЪБЕФpHжЕЃЌгІАбFe2ЃЋбѕЛЏЃЛМгШыCaOКЭHЃЋЗДгІЃЌдіДѓpHЕН5.2ЁЋ6.0ПЩЪЙAl3ЃЋЁЂFe3ЃЋЭъШЋзЊЛЏЮЊAl(OH)3ЁЂFe(OH)3ГСЕэЃЌГ§ШЅFe3ЃЋЁЂAl3ЃЋЃЛЕїНкpHжЕЪБЃЌМгШыСЫCaOЃЌЮЊСЫГ§ШЅCa2ЃЋЃЌгІМгШыNaFЃЌЪЙжЎЩњГЩCaF2ГСЕэЁЃ
(3)ШмвКAжаЕФвѕРызгЮЊSO42ЁЊЃЌЫљвдМгNH4HCO3КѓЃЌЩњГЩЕФжївЊЮяжЪЪЧ(NH4)2SO4ЃЌзїЮЊЕЊЗЪгУЁЃ
(4)Mn3O4ЃЋ2H2SO4=2MnSO4ЃЋMnO2ЃЋ2H2OЁЁ(гУЕЙХфЗЈХфЦНЃЌMn3O4жаMnЕФЛЏКЯМлЮЊЃЋ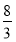 )ЁЃ
)ЁЃ

 УћаЃПЮЬУЯЕСаД№АИ
УћаЃПЮЬУЯЕСаД№АИ