��Ŀ����
����Ŀ��ij��Һ�п��ܺ�������6�������е�ij���֣�Cl��![]() ��
��![]() ��
��![]() ��K+��Na+��Ϊȷ����Һ��ɽ�������ʵ�飺
��K+��Na+��Ϊȷ����Һ��ɽ�������ʵ�飺
��200 mL������Һ����������BaCl2��Һ����Ӧ�������ˡ�ϴ�ӡ�����ó���4.30 g��������м�����������ᣬ��2.33 g�������ܡ�
����������Һ�м���������NaOH��Һ�����ȣ������ܴ�ʹʪ���ɫʯ����ֽ��������
��1.12 L(�ѻ���ɱ�״�����ٶ�����������ȫ���ݳ�)��
(1)��Һһ�����ڵ������� �����ܴ��ڵ������� ��
(2)ԭ��Һ��c(![]() )Ϊ ��c(
)Ϊ ��c(![]() ) c(
) c(![]() ) (���������=��)��
) (���������=��)��
(3)�������6�����Ӷ����ڣ���c(Cl) c(![]() ) (���������=��)��
) (���������=��)��
���𰸡�(1)![]() ��
��![]() ��
��![]() Cl��K+��Na+
Cl��K+��Na+
(2)0.05 mol/L ��
(3)��
����������ȡ��������Һ����BaCl2��Һ�а�ɫ�������ɣ��ټ�������������������ܽ⣬�����������ɣ�˵����ɫ����ΪBaCO3��BaSO4������һ����4.3 g������Һ�к���![]() ��
��![]() ��������м�����������ᣬ��2.33 g�������ܣ������ᱵ��������2.33 g��������������ӵ����ʵ�����
��������м�����������ᣬ��2.33 g�������ܣ������ᱵ��������2.33 g��������������ӵ����ʵ�����![]() =0.01 mol������̼�ᱵ��������4.3 g2.33 g=1.97 g��
=0.01 mol������̼�ᱵ��������4.3 g2.33 g=1.97 g��![]() �����ʵ�����
�����ʵ�����![]() =0.01 mol������������Һ�м���������NaOH��Һ�����ȣ�������ʹʪ���ɫʯ����ֽ�����������ǰ��������ʵ�����
=0.01 mol������������Һ�м���������NaOH��Һ�����ȣ�������ʹʪ���ɫʯ����ֽ�����������ǰ��������ʵ�����![]() =0.05 mol��˵����Һ����
=0.05 mol��˵����Һ����![]() �����ʵ�����0.05 mol���ۺ����Ϸ�����
�����ʵ�����0.05 mol���ۺ����Ϸ�����
(1)��Һһ�����ڵ������У�![]() ��
��![]() ��
��![]() �����ܴ��ڵ������У�Cl��K+��Na+��
�����ܴ��ڵ������У�Cl��K+��Na+��
(2)���ݼ���ó�c(![]() )=
)=![]() =0.05 mol/L��
=0.05 mol/L��![]() Ϊ0.01 mol��
Ϊ0.01 mol��![]() Ϊ0.05 mol����c(
Ϊ0.05 mol����c(![]() )��c(
)��c(![]() )��
)��
(3)������Һ������ԭ���趼���ڣ���ô0.05 mol+n(Na+)+n(K+)=2��0.01 mol+2��0.01 mol+n(Cl)���ݴ˵ó�n(Cl)=n(Na+)+n(K+)+0.01 mol��0.01 mol��

����Ŀ����������ʵ���ʵ��������������ý�����ȷ����
ʵ���ʵ����� | ���� | ʵ����� | |
A | �ô���ʯ�����ᷴӦ��ȡCO2���壬����ͨ��һ��Ũ�ȵ�Na2SiO3��Һ�� | ���ְ�ɫ���� | H2CO3�����Ա�H2SiO3������ǿ |
B | ��ij��Һ�ȵμ������ữ���ٵμ�BaCl2��Һ | �а�ɫ�������� | ԭ��Һ�к���SO42-��SO32-��HSO3-�е�һ�ֻ��� |
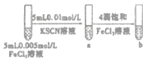
C |
| �Թ�b���Թ�a����Һ�ĺ�ɫ�� | ����Ӧ��Ũ�ȣ�ƽ��������Ӧ�����ƶ� |
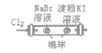
D |
| ��������Ϊ��ɫ���ұ������Ϊ��ɫ | �����ԣ�Cl2>Br2>I2 |
A. A B. B C. C D. D