题目内容
【题目】已知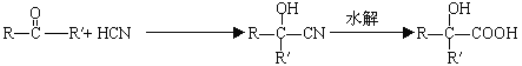 。分析下图变化,试回答下列问题:(E没有支链)
。分析下图变化,试回答下列问题:(E没有支链)

(1)写出下列有机物的结构简式:
A___________________C _______________________E ____________________F_________________。
(2)写出下列有关反应的化学方程式:
C →D_______________________;
D →E_______________________;
E→F_______________________。
【答案】 ![]()
![]() CH3-CH=CHCOOCH3
CH3-CH=CHCOOCH3 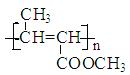
![]()
![]() CH3CH=CH-COOH+H2O CH3CH=CH-COOH+CH3OH
CH3CH=CH-COOH+H2O CH3CH=CH-COOH+CH3OH![]() CH3-CH=CHCOOCH3+H2O n CH3-CH=CHCOOCH3
CH3-CH=CHCOOCH3+H2O n CH3-CH=CHCOOCH3![]()
【解析】已知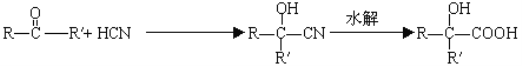 。分析下图变化,试回答下列问题:(E没有支链)
。分析下图变化,试回答下列问题:(E没有支链)

(1)根据A生成B的条件结合信息和E没有支链可知,A发生信息中反应生成 B,根据A的分子式可知,A为CH3CH2CHO,A与HCN发生加成反应生成B为CH3CH2CHOHCN,B水解得C为CH3CH2CHOHCOOH,C在浓硫酸作用下加热发生消去反应得D为CH3CH=CHCOOH,D发生酯化反应生成E,E为CH3CH=CHCOOCH3,E发生加聚反应得F为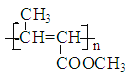 。综上所述,A为CH3CH2CHO,C为CH3CH2CHOHCOOH,E为CH3CH=CHCOOCH3,F为
。综上所述,A为CH3CH2CHO,C为CH3CH2CHOHCOOH,E为CH3CH=CHCOOCH3,F为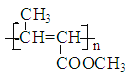 ,故答案为:CH3CH2CHO;CH3CH2CHOHCOOH;CH3CH=CHCOOCH3;
,故答案为:CH3CH2CHO;CH3CH2CHOHCOOH;CH3CH=CHCOOCH3;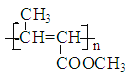 ;
;
(2)C →D的化学方程式为![]()
![]() CH3CH=CH-COOH+H2O;D →E的化学方程式为CH3CH=CH-COOH+CH3OH
CH3CH=CH-COOH+H2O;D →E的化学方程式为CH3CH=CH-COOH+CH3OH![]() CH3-CH=CHCOOCH3+H2O;E→F的化学方程式为CH3-CH=CHCOOCH3
CH3-CH=CHCOOCH3+H2O;E→F的化学方程式为CH3-CH=CHCOOCH3![]()
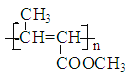 ;故答案为:
;故答案为: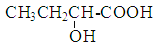
![]() CH3CH=CH-COOH+H2O;CH3CH=CH-COOH+CH3OH
CH3CH=CH-COOH+H2O;CH3CH=CH-COOH+CH3OH![]() CH3-CH=CHCOOCH3+H2O;n CH3-CH=CHCOOCH3
CH3-CH=CHCOOCH3+H2O;n CH3-CH=CHCOOCH3![]()
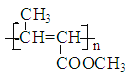 。
。

 阶梯计算系列答案
阶梯计算系列答案