��Ŀ����
ij��Һ�п��ܺ�������5�������е�ij���֣�Na+��NH4+��Mg2+��Al3+��Cl����Ϊȷ�ϸ���Һ��ɽ�������ʵ�飺��ȡ20.0 mL����Һ������25.0 mL 4.00 mol��L-1NaOH��Һ���а�ɫ���������ݼ���ζ���塣���ˡ�ϴ�ӡ�����ó���1.16 g���ٽ���Һϡ����100 mL�������Һ��c(OH��)Ϊ0.20 mol��L-1������ȡ20.0 mL����Һ������������AgNO3��Һ�����ɰ�ɫ����11.48 g���ɴ˿ɵó�����ԭ��Һ��ɵ���ȷ������
| A��һ������Mg2+��Al3+��Cl��������Na+��NH4+ |
| B��һ������Na+��Mg2+��Cl��������NH4+�����ܺ���Al3+ |
| C��c (Cl��) Ϊ 4.00 mol��L-1��c (Al3+) Ϊ1.00 mol��L-1 |
| D��c (Mg2+) Ϊ 1.00 mol��L-1��c(Na+ ) Ϊ 0.50 mol��L-1 |
D
�����������������ʵ����жϣ�ԭ��Һ��һ����Mg2+����NH4+���μӷ�Ӧ��OH����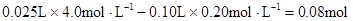 ��n(Mg2+)=
��n(Mg2+)=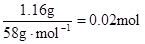 Mg2+ + 2OH��= Mg(OH)2������Mg2+��Ӧ��OH����
Mg2+ + 2OH��= Mg(OH)2������Mg2+��Ӧ��OH����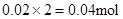 ������ԭ��Һ��һ������Al3+����Al3+��Ӧ��OH����
������ԭ��Һ��һ������Al3+����Al3+��Ӧ��OH����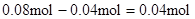 ������Һ�л���OH����������Ӧ Al3+ + 4OH��= AlO2�� + 2H2O ��n(Al3+ )=
������Һ�л���OH����������Ӧ Al3+ + 4OH��= AlO2�� + 2H2O ��n(Al3+ )=  ����ʵ��ڽ������ļ������ݣ��� n(Cl��)=
����ʵ��ڽ������ļ������ݣ��� n(Cl��)= 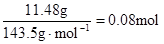 �� n(Cl��)��2n(Mg2+) + 3n(Al3+ ) ����ԭ��Һ�л����� Na+ ��2n(Mg2+) + 3n(Al3+ )+ n(Na+ ) =
�� n(Cl��)��2n(Mg2+) + 3n(Al3+ ) ����ԭ��Һ�л����� Na+ ��2n(Mg2+) + 3n(Al3+ )+ n(Na+ ) =  n(Na+ ) =
n(Na+ ) = 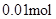
ԭ��Һ�����ӵ�Ũ�ȣ�
c (Cl��) = 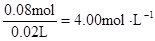 c (Al3+)=
c (Al3+)=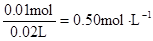
c (Mg2+)=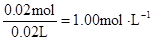 c(Na+ )=
c(Na+ )= 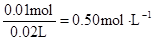
��ѡD��
���㣺���⿼��ѧ���������ӵļ��鷽���������Ѷȵļ��㣬���Ը�����ѧ��֪ʶ���ش��Ѷ��С�

��1����Ϊ�ڢ�A��Ԫ�أ����ĵ��ʺͻ�������ijЩ���ʵĻ�ѧ��������������֮������֪��Ԫ�ؾ����������ʣ�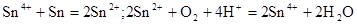 ��
��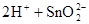

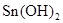

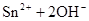 ��
��
�Իش�
�����������ᣬ����Ӧ�����Һ��ͨ���������йط�Ӧ������������Ӧ�仯����д���йط�Ӧ�Ļ�ѧ����ʽ��_____________________________________________________��_______________________________________��
�ڽ�������Һ���ɺ�����������ù��壬�仯����������FeCl3��Һ��Ӧ�ı仯�������õ��Ĺ���������__________���ѧʽ����
��������SnCl2��Һ������ļ���Һ��Ӧ�ķ�����Sn��OH��2���ü��ѡ��________��
��2��ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֡�
| ������ | 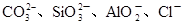 |
| ������ | 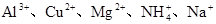 |
��ȡ����Һ�����������м���ij�Լ�Y���������������ʵ�����n��������Լ�Y�����V���Ĺ�ϵ��ͼ��ʾ��
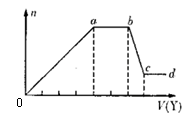
����Y�����ᣬ����Һ�к��еĽ�����������_________��ab�η�����Ӧ�����ӷ���ʽΪ_______________��ͼ��oa�βμӷ�Ӧ�������ӵ����ʵ���֮��Ϊ___________________��
����Y��NaOH��Һ����bc�η�Ӧ�����ӷ���ʽΪ____________________��
�����������ӵ�ˮ�⣬����H����OH����Ӱ�죬����Һ��ֻ�����������ӣ������ǵ����Ӹ�����Ϊ_____________________________________________������������ǰ���������ں���ǰ���ͼ��ں��˳�����У���
���и�����������Һ���ܴ����������
A��Na����Al3����Cl����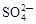 | B��Cu2����Cl����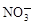 ��OH�� ��OH�� |
C��Ca2����Na����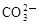 �� ��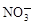 | D��H����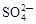 �� ��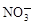 ��OH�� ��OH�� |
�������ӷ���ʽ��д��ȷ���ǣ� ��
| A������з�̪�Ĺ�������Һ�бӱ��صμ���������ɫ��dz���ӽ���ʧ 2H++SiO32��=H2SiO3�����壩 |
B���ö��Ե缫���MgCl2��Һ��2Cl��+2H2O Cl2��+H2��+2OH�� Cl2��+H2��+2OH�� |
C��̼�������Һ��������NaOH��Һ��Ϻ���ȣ� NH4����OH�� NH3����H2O NH3����H2O |
| D��5��6 g Fe��200 mL 2��0 mol/L HNO3��Һ��ַ�Ӧ��3Fe + 2NO3��+ 8H+��3Fe2+ + 2NO�� + 4H2O |
���£����и���������ָ����Һ���ܴ����������( )
| A��pH=1����Һ��: I-��NO3����SO42����Na�� |
| B����ˮ�����c(H+)=1��10-14 mol��L-1����Һ�У�Ca2����K����Cl����HCO3�� |
| C��c(H+)��c(OH��)=1012����Һ�У� NH4����Al3����NO3����Cl�� |
| D��c(Fe3��)="0.1" mol��L-1����Һ�У�K����ClO����SO42����SCN�� |
����Һ����ˮ���������c(OH��)��1��10��14 mol��L��1���������������Һ��һ�����Դ����������������( )
| A��Al3�� Na�� NO3�� Cl�� | B��K�� Na�� Cl�� NO3�� |
| C��K�� Na�� Cl�� AlO2�� | D��K�� NH4�� SO42�� NO3 |
���и�����������������Һ��ķ�Ӧ������ͬһ���ӷ���ʽ��ʾ����
| A��KCl+AgNO3��AlCl3+ AgNO3 |
| B��NaHCO3+H2SO4��Na2CO3+HCl |
| C��NaHCO3+NaOH��Ca��HCO3��+KOH |
| D��BaCl2+H2SO4��Ba(OH)2+H2SO4 |
����ȷ��ʾ���з�Ӧ�����ӷ���ʽΪ
| A��̼��������Һ�е�������������Һ��HCO3�D + OH�D �� CO32�D�� H2O |
| B����������ͨ�����������Һ��SO2 + ClO�D + 2OH�D�� SO42�D��Cl�D��H2O |
| C��������ϡ���BaS + 2H���� H2S��+ Ba2+ |
| D����������Һ�������Һ��ϣ�SiO32��+2H����H2SiO3�� |
�������ӷ���ʽ��ȷ����
| A����ˮ����������SO2���壺OH����SO2��HSO3�� |
| B����ͭ˿Ͷ��ϡ�����У�Cu��4H����NO3����Cu2����NO2����2H2O |
| C����H2O2��������KMnO4��Һ�У�2MnO4��+10H++3H2O2��2Mn2++3O2��+8H2O |
| D��NaHSO4��Һ�м������Ba(OH)2��Һ��H����SO42����Ba2����OH����BaSO4����H2O |