��Ŀ����
��1��
Ϊ���Т����еij���ʵ�飨������Ϊ���ʣ�����Ӣ�����ѡ��һ���Լ����Ӣ�����ѡ��һ���ʵ�ʵ������������룬��������ں���Ĵ����ڡ�
| �� ����ʵ�� | �� �Լ� | �� �������� | �� | ||
| �� | �� | �� | |||
| �ٱ������ӣ� | A������Na2CO3��Һ B��NaCl���� C��NaOH��Һ D��CaO | a������ b����Һ c������������ | �� | | |
| ���Ҵ���ˮ�� | �� | | | ||
| �۷��������͡�ˮ�� | �� | | | ||
| ���������������ᣩ | �� | | | ||
��
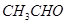 ��
�� 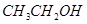 ��
��  ��
�� 
������
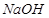 ��Һ��Ӧ����_________________��
��Һ��Ӧ����_________________��������������Һ��Ӧ������������____________________��
�ۼ��ܷ�����ȥ��Ӧ�����ܷ���������Ӧ����___________________��
�ܺ˴Ź�������ͼ����3�����շ壬�����Ϊ3��2��1����___________��
��3����4�֣�a.�ڽྻ���Թ��м���������������Һ������εμ�ϡ��ˮ�����������ɰ�ɫ��������Ӧ�����ӷ���ʽ�Ǣ�_______________________ �������μ�������ǡ���ܽ⣬����������Һ��b. ��a�����õ�������Һ�е���������������Һ����Ͼ��Ⱥ���ˮԡ�м���3min��5min���������Թ��ڱ����γ���������˷�Ӧ�Ļ�ѧ����ʽΪ�ڣ������ǵĽṹ��ʽ��G-CHO��ʾ���� ��
(1)��C b ��D a ��B c ��A b (2)�ۢ�; �٢�; ��; �ڢܡ�
(3)��Ag++NH3��H2O��AgOH��+NH4+ ��GCHO+2Ag(NH3)2OH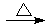 GCOONH4+2Ag��+3NH3+H2O
GCOONH4+2Ag��+3NH3+H2O
�������������(1)�ٱ����ܺ�����������Һ��Ӧ�����Գ�ȥ���б��ӵ��Լ�������������Һ��Ȼ���Һ���ɣ���ѡC��b��
���Ҵ���ˮ���ܣ�Ӧ������ʯ�ң�Ȼ�����ɣ���ѡD��a��
�������Ȼ��ƿ��Խ���֬�����Ƶ��ܽ�ȣ��Ӷ�������������ѡB��c��
����������������ˮ���������ܺͱ���̼������Һ��Һ�����Գ�ȥ����������������Լ���A�������Ƿ�Һ����ѡb��
��2�����Ȼ��������ܺ�����������Һ��Һ�����Դ�ѡ�ۢܡ�
��ȩ���ܷ���������Ӧ����������������Һ��Ӧ�����������Ǣ٢ܡ�
�۴��ǻ����ܷ�����ȥ��Ӧ�����ܷ���������Ӧ�����Դ�ѡ�ڡ�
������ͬһ��̼ԭ���ϵ���ԭ������ͬ�ģ����ͬһ��̼ԭ�������ӵ����м��ϵ���ԭ������ͬ�ģ��پ��Ǿ��жԳ��Խṹ�ģ�������ƽ�澵�������������Ĺ�ϵ�������Ժ˴Ź�������ͼ����3�����շ壬�����Ϊ3��2��1���Ǣڢܡ�
��3�����������ܺͰ�ˮ��Ӧ��������������������Ӧ�����ӷ���ʽ��Ag++NH3��H2O��AgOH��+NH4+��
�������Ƿ����к���ȩ�����ܷ���������Ӧ����Ӧ�Ļ�ѧ����ʽ��GCHO+2Ag(NH3)2OH GCOONH4+2Ag��+3NH3+H2O��
GCOONH4+2Ag��+3NH3+H2O��
���㣺�������ʵķ�����ᴿ���л���������Լ�������Ӧ
�����������Ǹ߿��еij������ͺͿ��㣬�����е��Ѷȵ����⡣���������߿����ۺ���ǿ����ע�ض�ѧ������֪ʶ������ѵ����ͬʱ�����ض�ѧ����������������ⷽ����ָ����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ���������������淶�Ͻ���ʵ������������Ĺؼ��Ǽ�ס���������ŵĽṹ�����ʣ�Ȼ���Ͻṹ��ʽ������������ü��ɡ�

 ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�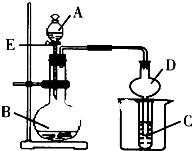 ij����С��������ͼװ�ý������ʵ�飬��ش��������⣮
ij����С��������ͼװ�ý������ʵ�飬��ش��������⣮