��Ŀ����
һ�ȼ����ijЩ�����������±���
��ʵ�����������ͼװ���Ʊ�һ�ȼ��飺
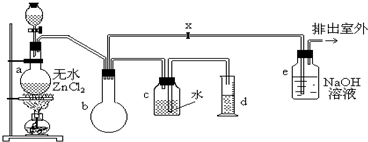
�Ʊ�װ�õķ�Һ©����ʢ�м״�����ƿ��ʢ��Ũ���ᣮ
����д���пհף�
��1���Ʊ�һ�ȼ���Ļ�ѧ����ʽ��______���÷�Ӧ����______��Ӧ��
��2��װ��e�п��ܷ�����Ӧ�Ļ�ѧ����ʽ��______��
��3�������������CH3Cl�ķ����ǣ���e���ݳ��ڵ�ȼCH3Cl���壬���������ɫ������������ȼ����������������������������HCl����CH3Clȼ�յĻ�ѧ����ʽ��______��
��4��װ��b��������______��ijѧ���ڹرջ���x�����������ʵ��ʱ���֣��ռ���һ�������������������ĵļ״���Ũ������������ۼ�������ࣨװ�õ�������û�����⣩����ԭ����______��ʵ�������d���ռ�����Һ���к���______��
| �۵� �� |
�е� �� |
Һ̬ʱ �ܶ� g/cm3 |
18��ʱ��ˮ�е��ܽ�� mL/L |
���ѡ���ͪ ���е� �ܽ��� |
�Ҵ� �е� �ܽ��� |
| -97.73 | -24.2 | 0.9159 | 280 | ���� | ���� |

�Ʊ�װ�õķ�Һ©����ʢ�м״�����ƿ��ʢ��Ũ���ᣮ
����д���пհף�
��1���Ʊ�һ�ȼ���Ļ�ѧ����ʽ��______���÷�Ӧ����______��Ӧ��
��2��װ��e�п��ܷ�����Ӧ�Ļ�ѧ����ʽ��______��
��3�������������CH3Cl�ķ����ǣ���e���ݳ��ڵ�ȼCH3Cl���壬���������ɫ������������ȼ����������������������������HCl����CH3Clȼ�յĻ�ѧ����ʽ��______��
��4��װ��b��������______��ijѧ���ڹرջ���x�����������ʵ��ʱ���֣��ռ���һ�������������������ĵļ״���Ũ������������ۼ�������ࣨװ�õ�������û�����⣩����ԭ����______��ʵ�������d���ռ�����Һ���к���______��
��1���״����Ȼ��ⷴӦ����һ�ȼ����ˮ��CH3OH+HCl��CH3Cl+H2O���״��е��ǻ�����ԭ�����������������ȡ����Ӧ���ʴ�Ϊ��CH3OH+HCl��CH3Cl+H2O��ȡ����
��2��һ�ȼ������������Ƶ�ˮ��Һ��ˮ�����ɼ״����Ȼ��������������Ʒ����кͷ�Ӧ���ʴ�Ϊ��CH3Cl+NaOH��CH3OH+NaCl��HCl+NaOH��NaCl+H2O��
��3��һ�ȼ�����������Ӧ���ɶ�����̼��ˮ���Ȼ��⣬�ʴ�Ϊ��2CH3Cl+3O2��2CO2+2H2O+2HCl��
��4�����������Ȼ��⣬���ܷ�����������bΪ��ȫƿ���ֹ�������÷�Ӧ�ǿ��淴Ӧ�����ܽ��е��ף���Ӧ����һ�ȼ��黹�����й������Ȼ��⣬
�ʴ�Ϊ����ȫƿ���ֹ�������÷�Ӧ�ǿ��淴Ӧ�����ܽ��е��ף�CH3Cl��HCl��
��2��һ�ȼ������������Ƶ�ˮ��Һ��ˮ�����ɼ״����Ȼ��������������Ʒ����кͷ�Ӧ���ʴ�Ϊ��CH3Cl+NaOH��CH3OH+NaCl��HCl+NaOH��NaCl+H2O��
��3��һ�ȼ�����������Ӧ���ɶ�����̼��ˮ���Ȼ��⣬�ʴ�Ϊ��2CH3Cl+3O2��2CO2+2H2O+2HCl��
��4�����������Ȼ��⣬���ܷ�����������bΪ��ȫƿ���ֹ�������÷�Ӧ�ǿ��淴Ӧ�����ܽ��е��ף���Ӧ����һ�ȼ��黹�����й������Ȼ��⣬
�ʴ�Ϊ����ȫƿ���ֹ�������÷�Ӧ�ǿ��淴Ӧ�����ܽ��е��ף�CH3Cl��HCl��

��ϰ��ϵ�д�
 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
�����Ŀ
һ�ȼ����ijЩ�����������±���
��ʵ�����������ͼװ���Ʊ�һ�ȼ��飺
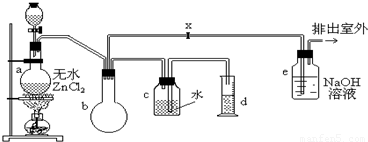
�Ʊ�װ�õķ�Һ©����ʢ�м״�����ƿ��ʢ��Ũ���ᣮ
����д���пհף�
��1���Ʊ�һ�ȼ���Ļ�ѧ����ʽ��______���÷�Ӧ����______��Ӧ��
��2��װ��e�п��ܷ�����Ӧ�Ļ�ѧ����ʽ��______��
��3�������������CH3Cl�ķ����ǣ���e���ݳ��ڵ�ȼCH3Cl���壬���������ɫ������������ȼ����������������������������HCl����CH3Clȼ�յĻ�ѧ����ʽ��______��
��4��װ��b��������______��ijѧ���ڹرջ���x�����������ʵ��ʱ���֣��ռ���һ�������������������ĵļ״���Ũ������������ۼ�������ࣨװ�õ�������û�����⣩����ԭ����______��ʵ�������d���ռ�����Һ���к���______��
| �۵� �� | �е� �� | Һ̬ʱ �ܶ� g/cm3 | 18��ʱ��ˮ�е��ܽ�� mL/L | ���ѡ���ͪ ���е� �ܽ��� | �Ҵ� �е� �ܽ��� |
| -97.73 | -24.2 | 0.9159 | 280 | ���� | ���� |

�Ʊ�װ�õķ�Һ©����ʢ�м״�����ƿ��ʢ��Ũ���ᣮ
����д���пհף�
��1���Ʊ�һ�ȼ���Ļ�ѧ����ʽ��______���÷�Ӧ����______��Ӧ��
��2��װ��e�п��ܷ�����Ӧ�Ļ�ѧ����ʽ��______��
��3�������������CH3Cl�ķ����ǣ���e���ݳ��ڵ�ȼCH3Cl���壬���������ɫ������������ȼ����������������������������HCl����CH3Clȼ�յĻ�ѧ����ʽ��______��
��4��װ��b��������______��ijѧ���ڹرջ���x�����������ʵ��ʱ���֣��ռ���һ�������������������ĵļ״���Ũ������������ۼ�������ࣨװ�õ�������û�����⣩����ԭ����______��ʵ�������d���ռ�����Һ���к���______��


