��Ŀ����
��11�֣���Ȼ����һ�ֽྻ������������Դ����Ȼ����Ҫ�ɷ������������м���ռ���������
��1��������Ӿ���____________�ṹ����ṹʽ��___________��
��2����Ȼ����һ�ָ�Ч���ͺġ���ȾС�������Դ����֪1mol������ȫȼ������CO2�����ˮ�����ų�802KJ������������ͬ�����£�1mol������ȫȼ������CO2�����Һ̬ˮ���ų�������________802KJ�����������������������
��3��ͨ������£�����Ƚ��ȶ��������ض������£�����Ҳ�ᷢ��ijЩ��Ӧ����д�������ڹ�����������������Ӧ����һ�ȼ���Ļ�ѧ����ʽ__________________�÷�Ӧ����______________���Ӧ���ͣ���
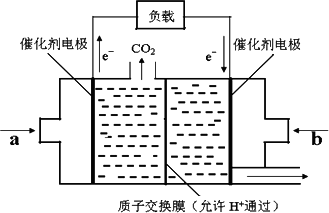
��4����һ�������¼���Ҳ������ȼ�ϵ�ء���ͼ�Ǽ���ȼ�ϵ�ص�ԭ��ʾ��ͼ��
�������ķ�ӦʽΪO2+4e����4H+��2H2O������ӦʽΪ___________________���õ�ع��������У�H�����ƶ�����Ϊ��_____��_____������ҡ�����
��11�֣���1���������壨1�֣���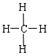 ��1�֣�����2������1�֣���
��1�֣�����2������1�֣���
��3��CH4+Cl2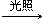 CH3Cl��HCl��2�֣���ȡ����Ӧ��1�֣���
CH3Cl��HCl��2�֣���ȡ����Ӧ��1�֣���
��4��CH4��8e����2H2O��CO2����8H+��3�֣������ң�ÿ��1�֣���2�֣�
����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�