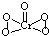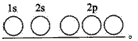��Ŀ����
�����������������̼���ڶͬԪ������ɵĺϽ�������Ҫ�ɷ�Ԫ�أ����ǵ�һ��Ҫ�ĺϽ�Ԫ�أ����и��ĺ������ܵ���11%����Ȼ�Ͳ���������������ĤCrO3��ֹ��ʴ����1��д��Fe2+�Ļ�̬���ӵĵ����Ų�ʽ��______����̬̼��C��ԭ�ӵĵ����Ų�ͼΪ______
��2��[Cr��H2O��4Cl2]Cl?2H2O��Cr����λ��Ϊ______����֪CrO5��CrΪ+6�ۣ���CrO5�ĽṹʽΪ______��
��3��H2O�ķ��ӹ���Ϊ______��H2O���Ӽ����γ������ԭ����______��
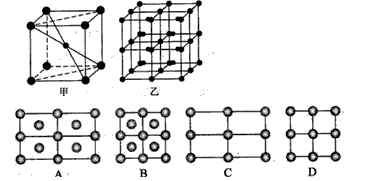
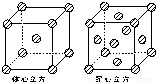
��4���������ľ����ڲ�ͬ�¶��������ֶѻ���ʽ�������ֱ���ͼ��ʾ������������������������������ʵ�ʺ��е�Feԭ�Ӹ���֮��Ϊ______��
���𰸡���������1������26��Ԫ�أ���ԭ��ʧȥ2�����ӱ���������ӣ������������Ӻ�����24�����ӣ����ݹ���ԭ��д�����������Ų�ʽ��̼ԭ�Ӻ�����6�����ӣ������������ԭ�������ﲻ����ԭ�������ع���д��������Ų�ͼ��
��2�����ݻ�ѧʽ�ж���λ����CrO5�д��ڹ�������Cr-O����Cr=O����4��Cr-O����CrΪ+1�ۣ���Cr=O��ע��CrΪ+2�ۣ��Դ���д�ṹʽ��
��3�����ݼ۲���Ӷ����ͦļ����жϷ��ӵ����幹�ͣ�Oԭ�ӵ縺�Խϴ��⻯���к��������
��4�����þ�̯�����㣮
����⣺��1��Fe��ԭ������Ϊ26��Fe�Ļ�̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p43s23p43d64s2����Fe2+�Ļ�̬���ӵĵ����Ų�ʽΪ[Ar]3d6��1s22s22p43s23p43d6��Cԭ������Ϊ6�����̬̼��C��ԭ�ӵĹ����ʾʽΪ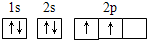 ��
��
�ʴ�Ϊ��[Ar]3d6��1s22s22p43s23p43d6��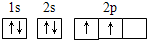 ��
��
��2��[Cr��H2O��4Cl2]Cl?2H2O��Cr��4��H2O��2��Cl-�γ���λ����������λ��Ϊ6��CrO5�д��ڹ�������Cr-O����Cr=O����4��Cr-O����CrΪ+1�ۣ���Cr=O����CrΪ+2�ۣ���ṹʽΪ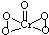 ���ʴ�Ϊ��6��
���ʴ�Ϊ��6��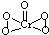 ��
��
��3��H2O�к���2���ļ���2���µ��Ӷԣ�����ӵ����幹��ΪV�Σ�Oԭ�ӵ縺�Խϴ��⻯���к��������
�ʴ�Ϊ��V�η��ӣ��������д��µ��Ӷԡ��縺�Խ�ǿ����ԭ�ӣ�������һ����縺�Խ�ǿ����ԭ��ֱ����������ԭ�ӣ�
��4��ͼ��������������Feԭ��λ�ڶ�������ģ������к���1+8× =2��Feԭ�ӣ�
=2��Feԭ�ӣ�
��������������Feԭ��λ�ڶ�������ģ������к���6× +8×
+8× =4��Feԭ�ӣ�
=4��Feԭ�ӣ�
�������־���Ļ����ṹ��Ԫ�е�ԭ�Ӹ���֮��Ϊ2��4=1��2��
�ʴ�Ϊ��1��2��
���������⿼���Ϊ�ۺϣ��漰�����Ų�ʽ���ṹʽ�Լ������ļ��㣬��Щ����ѧϰ�ص㼰�߿��ȵ㣬�ѵ���CrO5�Ľṹʽ��ȷ�����Ѷ��еȣ�
��2�����ݻ�ѧʽ�ж���λ����CrO5�д��ڹ�������Cr-O����Cr=O����4��Cr-O����CrΪ+1�ۣ���Cr=O��ע��CrΪ+2�ۣ��Դ���д�ṹʽ��
��3�����ݼ۲���Ӷ����ͦļ����жϷ��ӵ����幹�ͣ�Oԭ�ӵ縺�Խϴ��⻯���к��������
��4�����þ�̯�����㣮
����⣺��1��Fe��ԭ������Ϊ26��Fe�Ļ�̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p43s23p43d64s2����Fe2+�Ļ�̬���ӵĵ����Ų�ʽΪ[Ar]3d6��1s22s22p43s23p43d6��Cԭ������Ϊ6�����̬̼��C��ԭ�ӵĹ����ʾʽΪ
 ��
���ʴ�Ϊ��[Ar]3d6��1s22s22p43s23p43d6��
 ��
����2��[Cr��H2O��4Cl2]Cl?2H2O��Cr��4��H2O��2��Cl-�γ���λ����������λ��Ϊ6��CrO5�д��ڹ�������Cr-O����Cr=O����4��Cr-O����CrΪ+1�ۣ���Cr=O����CrΪ+2�ۣ���ṹʽΪ
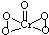 ���ʴ�Ϊ��6��
���ʴ�Ϊ��6��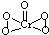 ��
����3��H2O�к���2���ļ���2���µ��Ӷԣ�����ӵ����幹��ΪV�Σ�Oԭ�ӵ縺�Խϴ��⻯���к��������
�ʴ�Ϊ��V�η��ӣ��������д��µ��Ӷԡ��縺�Խ�ǿ����ԭ�ӣ�������һ����縺�Խ�ǿ����ԭ��ֱ����������ԭ�ӣ�
��4��ͼ��������������Feԭ��λ�ڶ�������ģ������к���1+8×
 =2��Feԭ�ӣ�
=2��Feԭ�ӣ���������������Feԭ��λ�ڶ�������ģ������к���6×
 +8×
+8× =4��Feԭ�ӣ�
=4��Feԭ�ӣ��������־���Ļ����ṹ��Ԫ�е�ԭ�Ӹ���֮��Ϊ2��4=1��2��
�ʴ�Ϊ��1��2��
���������⿼���Ϊ�ۺϣ��漰�����Ų�ʽ���ṹʽ�Լ������ļ��㣬��Щ����ѧϰ�ص㼰�߿��ȵ㣬�ѵ���CrO5�Ľṹʽ��ȷ�����Ѷ��еȣ�

��ϰ��ϵ�д�
 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д� �������ϵ�д�
�������ϵ�д�
�����Ŀ