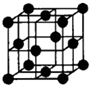
��Ŀ����
��֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A<B<C<D<E������A��B��C��ͬһ���ڵķǽ���Ԫ�ء�������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC Ϊ�Ǽ��Է��ӡ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߡ�E��ԭ�Ӻ�������Ų�δ�ɶԵ���������Ԫ�أ�ECl
Ϊ�Ǽ��Է��ӡ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߡ�E��ԭ�Ӻ�������Ų�δ�ɶԵ���������Ԫ�أ�ECl ����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����硣���������������ش��������⣺
����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����硣���������������ش��������⣺
������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
��1��A��B��C�ĵ�һ��������С�����˳��Ϊ ��
��2��A���⻯����һ�����ΪA2H2����,�˷���A��ԭ�ӹ�����ӻ�����Ϊ��������������1 mol A2H2���Ҽ�����ĿΪ����������
��3��д��������AC2�ĵ���ʽ ��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪ ��
��4��B��C���⻯��ķе��A���⻯��ķе�ߣ�����Ҫԭ������������
��5��E�ļ۵����Ų�ʽ�� ��ECl3�γɵ������Ļ�ѧʽΪ
��6��B������������Ӧ��ˮ�����ϡ��Һ��D�ĵ��ʷ�Ӧʱ��B����ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽ�� ��
(1)C<O<N (2) sp�ӻ���3 NA��3��6.02��1023��
(3) 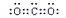 N
N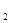 O (4) NH3 H
O (4) NH3 H O���Ӽ�������
O���Ӽ�������
(5)3d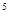 4S
4S ��Cr(NH3)4(H2O)2��Cl3
��Cr(NH3)4(H2O)2��Cl3
(6)4Mg+10HNO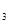 ="4" Mg(NO
="4" Mg(NO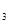 )
)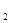 +NH
+NH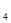 NO
NO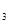 +3H
+3H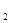 O
O
��������
���������D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ�����DΪMg��CΪO��A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AC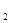 Ϊ�Ǽ��Է��ӣ���AΪC��BΪN; E��ԭ�Ӻ�������Ų�δ�ɶԵ���������Ԫ��,��������Ų�ʽΪ1s22s22p63s23p63d54s1����EΪCr��(1)ͬ����Ԫ�ص�һ�����ܴ���������������ƣ��ʵ����ܴ�СΪC<O<N����2��A2H2����Ϊ��Ȳ��̼ԭ����SP�ӻ���̼̼����Ϊһ���Ҽ��������м����ȵ�����ָ��������ԭ������ͬ�ķ��ӡ����ӻ���š���4�����ʵķе��С��Ҫ�ܷ��Ӽ�����������������ã�C��H֮�䣬H��O֮�䶼�����γ��������������ԶԶ���ڷ��Ӽ�����������5����ECl
Ϊ�Ǽ��Է��ӣ���AΪC��BΪN; E��ԭ�Ӻ�������Ų�δ�ɶԵ���������Ԫ��,��������Ų�ʽΪ1s22s22p63s23p63d54s1����EΪCr��(1)ͬ����Ԫ�ص�һ�����ܴ���������������ƣ��ʵ����ܴ�СΪC<O<N����2��A2H2����Ϊ��Ȳ��̼ԭ����SP�ӻ���̼̼����Ϊһ���Ҽ��������м����ȵ�����ָ��������ԭ������ͬ�ķ��ӡ����ӻ���š���4�����ʵķе��С��Ҫ�ܷ��Ӽ�����������������ã�C��H֮�䣬H��O֮�䶼�����γ��������������ԶԶ���ڷ��Ӽ�����������5����ECl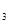 ����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����磬��֪�������Ϊ��Cr(NH3)4(H2O)2��Cl3����6��N����ͼ�Ϊ-3��
����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����磬��֪�������Ϊ��Cr(NH3)4(H2O)2��Cl3����6��N����ͼ�Ϊ-3��
���㣺ԭ�ӽṹ��Ԫ��������
������Ԫ�����ڱ���������Ǹ߿��ؿ�֪ʶ�㣬�����ڱ�����Ӧע��������ն�����Ԫ�صĽṹ�����ʡ�

 �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�| A��Fe��Cu��Al��Ag | B��Al��Cu��Fe��Ag | C��Cu��Ag��Al��Fe | D��Ag��Al��Cu��Fe |

 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ� ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�