��Ŀ����
N2O5��һ�������������������ʺ��Ʊ��ܵ����ǵĹ�ע��






��1��N2O5�뱽����������Ӧ���ɵ��������Ľṹ��ʽ�� ��
��2��һ���¶��£��ں����ܱ�������N2 O5�ɷ������з�Ӧ��
 2N2O5��g�� 4NO2��g��+O2(g);��H��0
2N2O5��g�� 4NO2��g��+O2(g);��H��0
�ٷ�Ӧ��ƽ�������ͨ��һ������������N2O5��ת���ʽ� ���������С���������䡱��
���±�Ϊ��Ӧ��T1�¶��µIJ���ʵ�����ݣ�
��500s��N2O5�ķֽ�����Ϊ ��
����T3�¶��£���Ӧ1 000 sʱ���NO2��Ũ��Ϊ4.98 mol��L-1����T2 T1��
��3������ͼ��ʾװ�ÿ������Ʊ�N2O5����N2O5�ڵ��ص�
�����ɣ���缫��ӦʽΪ .







��1��N2O5�뱽����������Ӧ���ɵ��������Ľṹ��ʽ�� ��
��2��һ���¶��£��ں����ܱ�������N2 O5�ɷ������з�Ӧ��
 2N2O5��g�� 4NO2��g��+O2(g);��H��0
2N2O5��g�� 4NO2��g��+O2(g);��H��0�ٷ�Ӧ��ƽ�������ͨ��һ������������N2O5��ת���ʽ� ���������С���������䡱��
���±�Ϊ��Ӧ��T1�¶��µIJ���ʵ�����ݣ�
| t/s | 0 | 500 | 1000 |
| c(N2O5)/mol��L-1 | 5.00 | 3.52 | 2.48 |
����T3�¶��£���Ӧ1 000 sʱ���NO2��Ũ��Ϊ4.98 mol��L-1����T2 T1��
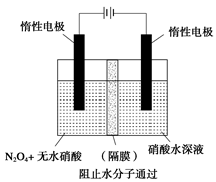 |
��3������ͼ��ʾװ�ÿ������Ʊ�N2O5����N2O5�ڵ��ص�
�����ɣ���缫��ӦʽΪ .
 ��1��
��1����2���ٲ���
��0.002 96 mol��L-1��s-1
�ۣ���С��
��3������ ��N2O4+2HNO3-2e-=2N2O5+2H+
��2������Ϊ�����³���N2��N2Ϊ�Ƿ�Ӧ�����壬����ƽ�ⲻ�ƶ������N2O5��ת���ʲ��䡣
��v(N2O5)=

="0.002" 96 mol��L-1��s-1��
��T1�¶���c (NO2)���㣺
 2N2O5(g) 4NO2(g)+O2(g);��H>0
2N2O5(g) 4NO2(g)+O2(g);��H>02 4
1 000 sʱ��5.00-2.48 c(NO2)
c(NO2)=2��(5.00-2.48) mol��L-1="5.04" mol��L-1>4.98 mol��L-1,���ڷ�Ӧ�����ȷ�Ӧ�����¶�Խ��NO2��Ũ��Խ�����Եõ�T2<T1��
��3����ͼ�и����������ʿ����ж�N2O5������N2O4�������ɣ�����N2O5�������������ݵ缫��Ӧ���ӷŵ�˳���֪����������2H++2e-��H2���ķ�Ӧ��������ΪN2O4+2HNO3-2e-��2N2O5+2H+��

��ϰ��ϵ�д�
 ��ҵ����ϵ�д�
��ҵ����ϵ�д�
�����Ŀ
 3S��+
3S��+
 O2��O
O2��O