��Ŀ����
��10�֣��ס��ҡ�����λͬѧ�ֱ�����������ʵ��װ�ü���ѧҩƷ�����м�ʯ��Ϊ�����������ƺ���ʯ�ҵĻ�����ȡ�������������̽�������ش��������⣺
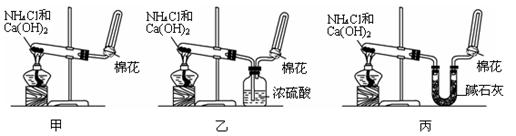
��1����λͬѧ��ȡ�����Ļ�ѧ����ʽΪ��_______________________
��2����λͬѧ���������ſ������ռ���������������ˮ������ԭ����_________
A������������ˮ B��������������ˮ
B��������������ˮ
C�������ܶȱȿ����� D�������ܶȱȿ���С
E�������ܶȱ�ˮ�� F�������ܶȱ�ˮС
��3����λͬѧ������װ����ȡ����ʱ,������ͬѧû���ռ����������ռ�������������Ҫԭ����____________________ (�û�ѧ����ʽ��ʾ)��
��4�����鰱���Ƿ��ռ����ķ�����_________
A���ŵ��а����ݳ�
B������������
C����ʪ��ĺ�ɫʯ����ֽ���Թܿڼ��飬������ֽ����
D����ʪ�����ɫʯ����ֽ���Թܿڼ��飬������ֽ���
��5����λͬѧ����Ϊ���ǵ�ʵ��װ��Ҳ�����ڼ���̼����粒�����ȡ�����İ��������ж��ܹ��ﵽʵ��Ŀ�ĵ���___________(��ס������ҡ�����)��

��1����λͬѧ��ȡ�����Ļ�ѧ����ʽΪ��_______________________
��2����λͬѧ���������ſ������ռ���������������ˮ������ԭ����_________
A������������ˮ
 B��������������ˮ
B��������������ˮC�������ܶȱȿ����� D�������ܶȱȿ���С
E�������ܶȱ�ˮ�� F�������ܶȱ�ˮС
��3����λͬѧ������װ����ȡ����ʱ,������ͬѧû���ռ����������ռ�������������Ҫԭ����____________________ (�û�ѧ����ʽ��ʾ)��
��4�����鰱���Ƿ��ռ����ķ�����_________
A���ŵ��а����ݳ�
B������������
C����ʪ��ĺ�ɫʯ����ֽ���Թܿڼ��飬������ֽ����
D����ʪ�����ɫʯ����ֽ���Թܿڼ��飬������ֽ���
��5����λͬѧ����Ϊ���ǵ�ʵ��װ��Ҳ�����ڼ���̼����粒�����ȡ�����İ��������ж��ܹ��ﵽʵ��Ŀ�ĵ���___________(��ס������ҡ�����)��
��1��2NH4Cl+Ca��OH��2====CaCl2+2NH3��+2H2O
��2��BD
��3��2NH3+H2SO4===��NH4��2SO4
��4��C
��5����
��2��BD
��3��2NH3+H2SO4===��NH4��2SO4
��4��C
��5����
��

��ϰ��ϵ�д�
�����Ŀ
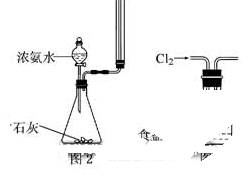
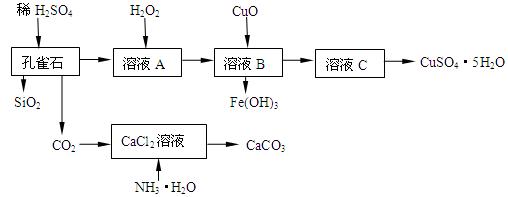
 ����CuCO3��Cu(OH)2��������������SiO2�����Ļ����ʵ�����Կ�ȸʯΪԭ���Ʊ�CuSO4��5H2O�IJ������£�
����CuCO3��Cu(OH)2��������������SiO2�����Ļ����ʵ�����Կ�ȸʯΪԭ���Ʊ�CuSO4��5H2O�IJ������£�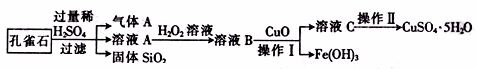
 �����ڴ˲����е���Ҫ������ ��
�����ڴ˲����е���Ҫ������ ��
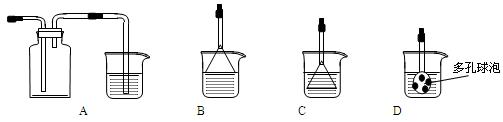
 �ܶ�Ϊ1.84 g��mL-1��_____________mL��
�ܶ�Ϊ1.84 g��mL-1��_____________mL�� ��һ��ʢ����������ˮ����Ͳ��ϡ�ͣ�����ȴ�����£�
��һ��ʢ����������ˮ����Ͳ��ϡ�ͣ�����ȴ�����£� �����ո����Բ��ӣ���
�����ո����Բ��ӣ���
 �������ƶ���ȷ����
�������ƶ���ȷ����