��Ŀ����
����Ŀ����ȩ��֬��Ӧ�ù㷺�ĸ߷��Ӳ��ϣ����÷�����ȩ��������Ĵ�������϶��ɣ����Ƶĺϳɹ����磺
��Ӧ�٣�
��Ӧ�ڣ�
��1��������I�ķ���ʽ____________��
��2��������Ũ��ˮ��Ӧ�Ļ�ѧ����ʽΪ___________________________��
��3�������� Ҳ����CH3CHO�������Ʒ�Ӧ�ٵķ�Ӧ�������л���÷�Ӧ��ѧ����ʽΪ_______________________________���л���������Ʒ�Ӧ�ڵķ�Ӧ�����ɸ߷��ӻ�����IV�Ľṹ��ʽΪ_____________________��
Ҳ����CH3CHO�������Ʒ�Ӧ�ٵķ�Ӧ�������л���÷�Ӧ��ѧ����ʽΪ_______________________________���л���������Ʒ�Ӧ�ڵķ�Ӧ�����ɸ߷��ӻ�����IV�Ľṹ��ʽΪ_____________________��
��4���йػ������͢��˵����ȷ����_________������ĸ����
A.�����ڷ�����
B.������FeCl3��Һ������ɫ��Ӧ
C.������NaHCO3��Һ��Ӧ�ų�CO2
D.l mol�Ģ�������2mol��NaOH��ȫ��Ӧ
���𰸡� C7H8O2 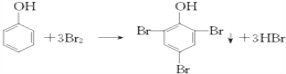
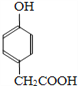 + CH3CHO
+ CH3CHO ![]()
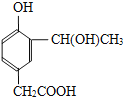
 BCD
BCD
����������1��������I�ķ���ʽC7H8O2 ����2��������Ũ��ˮ����ȡ����Ӧ����ѧ����ʽΪ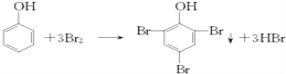 ����3��������
����3��������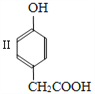 Ҳ����CH3CHO�������Ʒ�Ӧ�ٵļӳɷ�Ӧ������ʽΪ��
Ҳ����CH3CHO�������Ʒ�Ӧ�ٵļӳɷ�Ӧ������ʽΪ��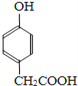 + CH3CHO
+ CH3CHO ![]()
 ���л���������Ʒ�Ӧ�ڵķ�Ӧ�����ɸ߷��ӻ�����IV�Ľṹ��ʽΪ
���л���������Ʒ�Ӧ�ڵķ�Ӧ�����ɸ߷��ӻ�����IV�Ľṹ��ʽΪ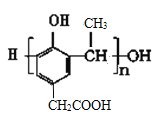 ��4���йػ������͢��˵��A.�����ڷ����廯�����C��H���OԪ�أ���A����B.�����з��ǻ���������FeCl3��Һ������ɫ��Ӧ����B��ȷ��C.������-COOH��������NaHCO3��Һ��Ӧ�ų�CO2����C��ȷ��D. l mol�Ģ�������2mol��NaOH��ȫ��Ӧ�����ǻ����Ȼ�������1molNaOH����D��ȷ����ѡBCD��
��4���йػ������͢��˵��A.�����ڷ����廯�����C��H���OԪ�أ���A����B.�����з��ǻ���������FeCl3��Һ������ɫ��Ӧ����B��ȷ��C.������-COOH��������NaHCO3��Һ��Ӧ�ų�CO2����C��ȷ��D. l mol�Ģ�������2mol��NaOH��ȫ��Ӧ�����ǻ����Ȼ�������1molNaOH����D��ȷ����ѡBCD��

 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д� ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�����Ŀ��
ѡ�� | ʵ��̽������ | ʵ����Ʒ |
A | ͭ˿��������ȼ�� | ����ǯ��ȼ�ճס�������ͭ˿ |
B | ���ȷ�Ӧ | ����̨������������ͨ©�������ۡ�Fe2O3 |
C | ʵ�����Ʊ����� | �Թܡ�����ƿ���ƾ��ơ�NH4Cl��Ca(OH)2 |
D | ��KMnO4��������500mL0.1mol��L��1KMnO4��Һ | ����ƿ���ձ�������������ʽ�ζ��ܡ�KMnO4 |
����Ŀ��ij��ѧС��ͬѧ������װ�ú��Լ�����ʵ�飬̽��O2��KI��Һ������Ӧ��������

��ѡ�Լ���30% H2O2��Һ��0.1mol/L H2SO4��Һ��MnO2���塢KMnO4����
��1��С��ͬѧ��Ƽס��ҡ�������ʵ�飬��¼���£�
���� | ���� | |
�� | ��I����ƿ�м���______��.��I��______�м���30% H2O2��Һ������I������ | I�в�����ɫ���岢�����������������������ð������ҺѸ�ٱ��� |
�� | ����м���KMnO4���壬���Ӣ�ȼ�ƾ��� | ����������ð������Һ������ |
�� | ����м���KMnO4���壬���м�������0.1mol/L H2SO4��Һ�����Ӣ�ȼ�ƾ��� | ����������ð������Һ���� |
��2����ʵ����O2��KI��Һ��Ӧ�����ӷ���ʽ��__________________________��
��3���Ա��ҡ���ʵ���֪��O2��KI��Һ������Ӧ������������___________��Ϊ��һ��̽���������Է�Ӧ���ʵ�Ӱ�죬�ɲ�ȡ��ʵ���ʩ��________��
��4���ɼס��ҡ�����ʵ���Ʋ⣬��ʵ�������I�еİ���ʹ��Һ������ѧ����I�в���������ֱ��ͨ������________��Һ(����ţ���֤���˰����к���H2O2��
A.���� KMnO4 B. FeCl2 C. Na2S D.Ʒ��
��5��������ʾ��KI��Һ�ڿ����о��ù����лᱻ����������4KI +O2 +2H2O=2I2 + 4KOH����С��ͬѧȡ20 mL���õ�KI��Һ�������м��뼸�ε�����Һ�����û�й۲쵽��Һ��ɫ���������Dz�������Ƿ����˷�Ӧ��д���ӷ���ʽ��________________________��ɵ�,�����ʵ��֤�����ǵIJ����Ƿ���ȷ________________________________________________��
����Ŀ��ú������Һ�����ִ���Դ��ҵ���ص㿼�ǵ���Դ�ۺ����÷������������������Ϊ��ú����ˮú��������ǰ�Ƚ����е�Һ������Ϊ��ú����CH3OH����֪�Ʊ��״����йػ�ѧ��Ӧ��ƽ�ⳣ��������
��CO2(g)��3H2(g) ![]() CH3OH(g)��H2O(g) ��H1����90.8 kJ��mol��1
CH3OH(g)��H2O(g) ��H1����90.8 kJ��mol��1
��CO(g)��H2O(g) ![]() CO2(g)��H2(g) ��H2����41.2 kJ��mol��1
CO2(g)��H2(g) ��H2����41.2 kJ��mol��1
��CO(g)��2H2(g) ![]() CH3OH(g)����H3
CH3OH(g)����H3
850 ��ƽ�ⳣ���ֱ�ΪK1��160��K2��243��K3��160���״����������ᷴӦ������CH3OH(l)��CH3COOH(l) ![]() CH3COOCH3(l)��H2O(l)��
CH3COOCH3(l)��H2O(l)��
(1)��Ӧ��H3��____________�������Ϸ�Ӧ��K�ı���ʽ________________��
(2)��CO�ϳɼ״�ʱ�������йظ÷�Ӧ��˵����ȷ����________(����ĸ)��
A���������������������������ڵ�ѹǿ�������仯������淴Ӧ�ﵽƽ��
B��һ����������H2 ������������CO���������ʵ�2��ʱ�����淴Ӧ�ﵽƽ��
C��ʹ�ú��ʵĴ��������̴ﵽƽ���ʱ�䲢���CH3OH �IJ���
D��ij�¶�������2 mol CO��6 mol H2 ����2 L�ܱ�����������ַ�Ӧ���ﵽƽ��������c(CO)��0.2 mol��L��1����CO��ת����Ϊ80%
(3)850 ��ʱ�����ܱ������н��з�Ӧ������ʼʱֻ����CO2��H2����Ӧ10 min���ø���ֵ�Ũ�����±����Ƚ������淴Ӧ�����ʵĴ�С��v��________(����������������������)v������ʱ����ڷ�Ӧ����v(H2)��____________________________________________________��
���� | H2 | CO2 | CH3OH | H2O |
Ũ��/mol��L��1 | 0.2 | 0.2 | 0.4 | 0.4 |
(4)��һ��������3 L�����ܱ�������������һ������H2��CO2��������Ӧ����ʵ���÷�Ӧ���ڲ�ͬ��ʼͶ����������Ӧ��ϵ��CO2��ƽ��ת�������¶ȵĹ�ϵ��������ͼ1��ʾ��
![]()

��H2��CO2����ʼ��Ͷ������A��B���ַ�ʽͶ��
A��n(H2)��3 mol��n(CO2)��1.5 mol��
B��n(H2)��3 mol��n(CO2)��2 mol����������������Ͷ�뷽ʽ________(��A��B��ʾ)��
�����¶�Ϊ500 K��������������A��ʽ����3 mol H2��1.5 mol CO2���÷�Ӧ10 minʱ�ﵽƽ�����ڴ���������ϵͳ��CH3OH��Ũ���淴Ӧʱ��ı仯������ͼ2��ʾ������Ӧʱ��ﵽ3 minʱ��Ѹ�ٽ���ϵ�¶�����600 K������ͼ2�л���3��10 min��������CH3OHŨ�ȵı仯����������_____________________



