��Ŀ����
����Ŀ��������X��һ���������ɲ�����ϩ��ױ�Ϊ��Ҫԭ����������·�ߺϳ���
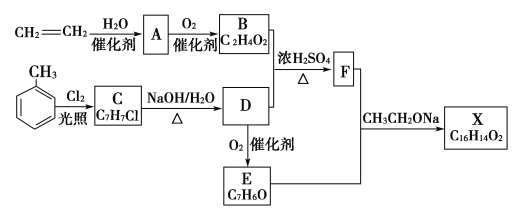
(1)д������ϩ��ȡA�Ļ�ѧ����ʽ��______________________________________________________��
(2)��ϩ��ʹ��ˮ������KMnO4��Һ��ɫ��������ɫԭ����ͬ��________��ԭ����_____________________________________________________________________��
(3)����ϩΪԭ�ϣ��ܷ��Ƶ���Ȳ��________�����ܣ���д����صĻ�ѧ����ʽ��_________________________________________________________________________________��
(4)��д��C�ĺ��б�����ͬ���칹��Ľṹ��ʽ��___________________________________��
(5)д���ױ���Ũ�����Ũ����Ļ���ᷴӦ�Ļ�ѧ����ʽ��________________________��
(6)�Լױ�Ϊ��˵���л������֮����Ӱ�죺_______________________��
(7)д��C��D�Ļ�ѧ����ʽ��__________________________________________���䷴Ӧ����Ϊ____________________________________��
(8)C�ܷ�����ȥ��Ӧ��________��ԭ����__________________________________________��
���𰸡�CH2===CH2��H2O![]() CH3CH2OH��ͬ��ϩʹ��ˮ��ɫ�����˼ӳɷ�Ӧ����ʹ����KMnO4��Һ��ɫ����������Ӧ��CH2===CH2��Br2�D��
CH3CH2OH��ͬ��ϩʹ��ˮ��ɫ�����˼ӳɷ�Ӧ����ʹ����KMnO4��Һ��ɫ����������Ӧ��CH2===CH2��Br2�D��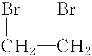 ��
�� ��2NaOH
��2NaOH![]() CH��CH����2NaBr��2H2O
CH��CH����2NaBr��2H2O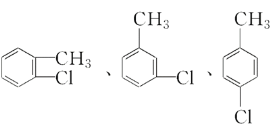
![]() ��3HNO3
��3HNO3![]()
 ��3H2O�ױ���������CH3�ͱ����Ӱ�죬�����Լ�����Ӱ�죬ʹ����CH3�ɱ�����KMnO4��������CH3�Ա�������Ӱ�죬ʹ��CH3�ڡ���λ��ԭ�Ӹ����á����ױ�ȡ��
��3H2O�ױ���������CH3�ͱ����Ӱ�죬�����Լ�����Ӱ�죬ʹ����CH3�ɱ�����KMnO4��������CH3�Ա�������Ӱ�죬ʹ��CH3�ڡ���λ��ԭ�Ӹ����á����ױ�ȡ��![]() ��NaOH
��NaOH![]()
![]() ��NaClˮ��(ȡ��)��Ӧ����
��NaClˮ��(ȡ��)��Ӧ����![]() ����ԭ������̼ԭ�ӵ���λ̼ԭ��������ԭ��
����ԭ������̼ԭ�ӵ���λ̼ԭ��������ԭ��
��������
�������֪����ϩ��ˮ�ڴ��������¼ӳ������Ҵ�����AΪ�Ҵ����Ҵ���һ��������ΪB�����BΪ���ᣬ�ױ����������յ������·���ȡ����Ӧ����C�����CΪһ�ȼױ�![]() ��C����������ˮ��Һ���������·���ȡ����Ӧ����DΪ
��C����������ˮ��Һ���������·���ȡ����Ӧ����DΪ![]() ��D��B����������Ӧ������F��D������Ϊ����ȩ��
��D��B����������Ӧ������F��D������Ϊ����ȩ��
��1����ϩ��ˮ�ڴ����������»������Ҵ�����ѧ����ʽΪ��CH2===CH2��H2O![]() CH3CH2OH��
CH3CH2OH��
��2����ϩ��ʹ��ˮ������KMnO4��Һ��ɫ��������ɫԭ������ͬ����ϩ��ʹ��ˮ��ɫ�Ƿ����˼ӳɷ�Ӧ����ϩʹ����KMnO4��Һ��ɫ������ϩ�����Ը��������Һ������
��3����ϩ���嵥�ʷ����ӳɷ�Ӧ����1,2-�������顢1,2-�����������������ƵĴ���Һ�Լ����ȵ������»ᷢ����ȥ������Ȳ����ѧ����ʽΪ��CH2===CH2��Br2�D��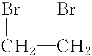 ��
��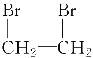 ��2NaOH
��2NaOH![]() CH��CH����2NaBr��2H2O ��
CH��CH����2NaBr��2H2O ��
��4��C�Ļ�ѧʽΪC7H7Cl��C���ɼױ�����ȡ����Ӧ���ɵģ���C�Ľṹ��ʽΪ![]() ����C��������ͬ���칹��Ϊ��
����C��������ͬ���칹��Ϊ��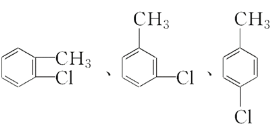 ��
��
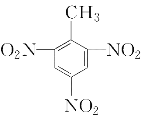
��5���ױ���Ũ�����Ũ����Ļ���ᷢ����Ӧ�����������ױ�����ѧ����ʽΪ��![]() ��3HNO3
��3HNO3![]()
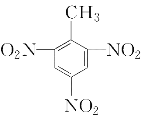 ��3H2O ��
��3H2O ��
��6���ױ�������-CH3�ͱ����Ӱ�죬������-CH3����Ӱ�죬ʹ��-CH3�ɱ�����KMnO4������-CH3�Ա�������Ӱ�죬ʹ-CH3�ڡ���λ��ԭ�Ӹ����á����ױ�ȡ����
��7��C���ɼױ�����ȡ����Ӧ���ɵģ���C�Ľṹ��ʽΪ![]() ��C���������Ƶ�ˮ��Һ�з���ˮ�ⷴӦ���ɱ��״���Ϊˮ�ⷴӦ��ˮ�ⷴӦҲ����ȡ����Ӧ����ѧ����ʽΪ��
��C���������Ƶ�ˮ��Һ�з���ˮ�ⷴӦ���ɱ��״���Ϊˮ�ⷴӦ��ˮ�ⷴӦҲ����ȡ����Ӧ����ѧ����ʽΪ��![]() ��NaOH
��NaOH![]()
![]() ��NaCl ��
��NaCl ��
��8��C���ɼױ�����ȡ����Ӧ���ɵģ���C�Ľṹ��ʽΪ![]() ������±��������������
������±��������������![]() ����ԭ������̼ԭ�ӵ���λ̼ԭ��������ԭ�ӣ����ܷ�����ȥ��Ӧ��
����ԭ������̼ԭ�ӵ���λ̼ԭ��������ԭ�ӣ����ܷ�����ȥ��Ӧ��

 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д� ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�����Ŀ��̼�͵��Ƕ�ֲ�����е���Ҫ���Ԫ�أ�������й����ŷŶ�����̼���������ЧӦ���������������⻯ѧ������Ŀǰ����Щ�ж��к�����Ĵ�����Ϊ��ѧ�о�����Ҫ���ݡ�
��1����֪�Ȼ�ѧ����ʽ����2C2H2(g)��5O2(g)===4CO2(g)��2H2O(l)����H1
��C(s)��O2(g)===CO2(g)����H2
��H2(g)��1/2O2(g)===H2O(l)����H3
��Ӧ��2C(s)��H2(g)===C2H2(g)�Ħ�H=_________��(�ú���H1����H2����H3�Ĺ�ϵʽ��ʾ)
��2������������Ӧ�����ȼ�ϵ�أ��������ҺΪ����������Һ����д����صĸ�����Ӧʽ��__________________________________________ ��
��3���û���̿��ԭ�����Դ����������ij�о�С����ij�ܱ������м���һ�����Ļ���̿��NO��������ӦC(s)��2NO(g)![]() N2(g)��CO2(g)����H<0����T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2(g)��CO2(g)����H<0����T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
ʱ��/min Ũ��/(mol��L��1) ���� | 0 | 10 | 20 | 30 | 40 | 50 |
NO | 1.00 | 0.68 | 0.50 | 0.50 | 0.60 | 0.60 |
N2 | 0 | 0.16 | 0.25 | 0.25 | 0.30 | 0.30 |
CO2 | 0 | 0.16 | 0.25 | 0.25 | 0.30 | 0.30 |
��10��20 min�ڣ�NO��ƽ����Ӧ����v(NO)��______��T1��ʱ���÷�Ӧ��ƽ�ⳣ��K��________��
��30 min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⣬�����ϱ��е������жϸı������������________(����ĸ���)��
a��ͨ��һ������NO b������һ������C c���ʵ����߷�Ӧ��ϵ���¶�
d��������ʵĴ��� e���ʵ���С���������
����������������Ӧǰ30 min�ķ�Ӧ������ͬ����ʼʱNO��Ũ��Ϊ2.50 mol��L��1����Ӧ��ƽ��ʱc(NO)��________��NO��ת���ʣ�________��