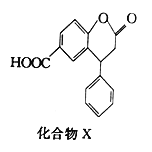
��Ŀ����
����Ŀ����1�����и����е������л����������ͬ�����ʡ�ͬϵ���ͬ���칹��ȣ����ж�����֮��Ĺ�ϵ
��2���������������_____________________________��
��1����ϩ�ͻ�����_______________________________��
��2��֧��ֻ��һ���һ�����Է���������С�������Ľṹ��ʽ____________________��
��3��д����ȩ��Һ��������������Һ���ȵ����ӷ���ʽ��_____________________________ ��
��4��д��1,3-����ϩ���嵥�ʷ���1,4-�ӳɵķ�Ӧ����ʽ__________________��
��5��д������ϩ���Ҵ��Ļ�ѧ����ʽ__________________________ ��
���𰸡� ͬ������ ͬ���칹�� ![]()
![]()
![]()
![]()
��������(1)��2-����������������ʽ��ΪC5H12���ҽṹ��ͬ����ͬ�����ʣ�
��1-��ϩ�ͻ�����ķ���ʽΪC6H12�������ʽ��ͬ���ṹ��ͬ����������ͬ���칹�壻
(2)�һ�������3��λ��ֻ��һ���һ������������ٺ���5��C����ʽ����С�������Ľṹ��ʽΪ��![]() ��
��
(3)��ȩ��������Һ��Ӧ���ɴ���李������ʡ�������ˮ����Ӧ�����ӷ���ʽΪ��CH3CHO+2Ag(NH3)2OH![]() CH3COONH4+H2O+2Ag��+3NH3��
CH3COONH4+H2O+2Ag��+3NH3��
(4)1��3-����ϩ���嵥�ʷ���1��4-�ӳɷ�Ӧ�ķ�Ӧ����ʽΪ��CH2=CH-CH=CH2+Br2��CH2BrCH=CHCH2Br��
(5)���Ҵ�һ���������飬�������Ҵ����廯�ⷢ��ȡ����Ӧ��ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ��CH3CH2OH+HBr![]() CH3CH2Br+H2O��
CH3CH2Br+H2O��

����Ŀ����������;�㷺,������ָʾ���ʹ������Ʊ���һ������ˮ�ܿ�[��Ҫ�ɷ�ΪCo2O3,������Fe2O3��Al2O3��MnO2��MgO��CaO��]��ȡ�����ܾ��壨CoC2O42H2O������������ͼ:
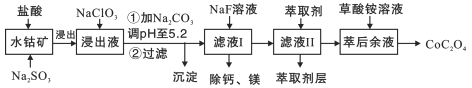
��֪���ٽ���Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Ca2+��Mg2+��Al3+�ȣ�
�ڲ���������������������ʽ����ʱ��Һ��pH���±���
������ | Fe(OH)3 | Fe(OH)2 | Co(OH)2 | Al(OH)3 | Mn(OH)2 |
��ȫ������PH | 3.7 | 9.6 | 9.2 | 5.2 | 9.8 |
(1)���������м���Na2SO3�����ӷ���ʽΪ________________________
(2)NaClO3��������_________________��PH��5.2����������Ҫ�ɷ�Ϊ__________
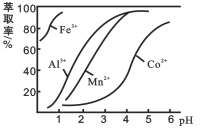
(3)��ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��ͼ2��ʾ����Һ���м�����ȡ����������___________________��ʹ����ȡ�����˵�pH��_______________
A. �ӽ�2.0 B.�ӽ�3.0 C.�ӽ�5.0
(4)���ơ�þ�ǽ���Һ��Ca2+��Mg2+ת��ΪMgF2��CaF2��������֪Ksp(MgF2)=7.35��1011��Ksp(CaF2)=1.05��1010.���������NaF��,������Һc(Mg2+)��c(Ca2+)=________________.
(5)��֪���ܷ����к�Co2O3��������Ϊa%,��ȡmkg�ú��ܷ��ϰ����������̣�������������Ƶò����ܾ��������Ϊ_________________g
����Ŀ����ҵȼ��ú����ʯ�͵Ȼ�ʯȼ���ͷų�������������(NOx)��CO2��SO2�����壬������Ⱦ�������� ����������������̼����������ʵ����ɫ����������������
��������
��֪��H2��ȼ����Ϊ285.8kJ��mol-1
N2(g)+2O2(g)=2NO2(g) ��H=+l33kJ��mol-1
H2O(g)=H2O(l) ��H=-44kJ��mol-1
�ڴ��������£�H2��ԭNO2������ˮ���������������ʵ��Ȼ�ѧ����ʽΪ��____________��
��.��̼����2L���ܱ������г���2mo1 CO2��6mol H2�����ʵ��Ĵ��������·�����Ӧ��
CO2(g)+3H2(g)![]() CH3OH(l)+H2O(l) ��H<0
CH3OH(l)+H2O(l) ��H<0
�ٸ÷�Ӧ�Է����е�������___________(������»������¶ȡ�)
������������˵���˷�Ӧ�ﵽƽ��״̬����________(����ĸ)
a.��������ƽ��Ħ���������ֲ���
b. CO2��H2������������ֲ���
c.CO2��H2��ת�������
d. ���������ܶȱ��ֲ���
e. 1mol CO2���ɵ�ͬʱ��3 mul H�DH������
��CO2��Ũ����ʱ��(0��t2)�仯����ͼ��ʾ����t2ʱ�������ݻ���Сһ����t3ʱ�ﵽƽ�⣬�뻭��t2��t4CO2Ũ����ʱ��ı仯��______

(2)�ı��¶ȣ�ʹ��ӦCO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) ��H<0�е��������ʶ�Ϊ��̬����ʼ�¶ȡ������ͬ��T1�桢2L�ܱ�����������Ӧ�����в������ݼ��±���
CH3OH(g)+H2O(g) ��H<0�е��������ʶ�Ϊ��̬����ʼ�¶ȡ������ͬ��T1�桢2L�ܱ�����������Ӧ�����в������ݼ��±���
��Ӧʱ�� | CO2(mol) | H2(mol) | CH3OH(mol) | H2O(mol) | |
��ӦI�����º��� | 0min | 2 | 6 | 0 | 0 |
l0min | 4.5 | ||||
20min | 1 | ||||
30min | 1 | ||||
��Ӧ���Ⱥ��� | 0min | 0 | 0 | 2 | 2 |
�� �ﵽƽ��ʱ����ӦI����Աȣ�ƽ�ⳣ��K(I)_____K(��)(�>����<����=����ͬ����ƽ��ʱCH3OH��Ũ�� c(I)______c(��)��
�ڶԷ�ӦI��ǰ10min�ڵ�ƽ����Ӧ����v(CH3OH)=_________���������������������£���30minʱֻ�ı��¶�T2�棬��ʱH2�����ʵ���Ϊ3.2mol����T1________T2(�>����<����=������
����Ŀ����ͼ��Ԫ�����ڱ���һ���֣������г��ˢ�����Ԫ�ص�λ�ã������Ҫ��ش����⡣
�� ���� | IA | ��A | ��A | ��A | VA | VIA | ��A | 0 |
�� | �� | �� | ||||||
�� | �� | �� | �� | �� | �� | �� | ||
�� | �� |
��1������ЩԪ���У���ѧ��������õ�ԭ�ӵ�ԭ�ӽṹʾ��ͼΪ________________��
��2��������γɵĻ�����ĵ���ʽ�ɱ�ʾΪ _________________________________________��
��3����ЩԪ�ص����{���������Ӧ��ˮ�����У�������ǿ����____________________���ѧʽ����
��4��д����������������Һ��Ӧ�Ļ�ѧ����ʽ��_____________________________________��