��Ŀ����
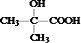
�л���Aֻ��C��H��O����Ԫ�أ�����Է�������Ϊ76��Ϊ�ⶨA �Ļ�ѧʽ��ȡ3.8 �˵�A�����ܱ���������ȫȼ�գ���ȼ�ղ���ͨ��ʢ��Ũ������Լ�ƿ���Լ�ƿ����3.6 �ˣ�����ͨ��ʢ�б�������������Һ���Լ�ƿ���Լ�ƿ����6.6�ˣ���A�ķ���ʽΪ______��
�ں��������ʾA������ֻ�С�C-C��������C-H�����͡�O-H�����������գ��ɾݴ��ƶ�A���ܵĽṹ��ʽΪ������֪��ͬһ��̼ԭ�������������ǻ��Dz��ȶ��Ľṹ��______��______��
����A�ĺ˴Ź�������ֻ���������շ壬��д��A�����������Ʒ�Ӧ�Ļ�ѧ����ʽ��______����д��A�����������ȣ�ͭ����������Ӧ�Ļ�ѧ����ʽ��______
���𰸡���������ͨ��Ũ��������3.6g��ˮ����������������������Һ����6.6�������ɶ�����̼������������n= ����ˮ��������̼�����ʵ������ٸ���ԭ���غ����Cԭ�ӡ���ԭ�ӵ����ʵ���������m=nM����Cԭ�ӡ���ԭ�ӵ����������������л�����Oԭ�ӵ�����������n=
����ˮ��������̼�����ʵ������ٸ���ԭ���غ����Cԭ�ӡ���ԭ�ӵ����ʵ���������m=nM����Cԭ�ӡ���ԭ�ӵ����������������л�����Oԭ�ӵ�����������n= ������ԭ�ӵ����ʵ�����ȷ�����ʽ�������Է�������������л���ķ���ʽ��
������ԭ�ӵ����ʵ�����ȷ�����ʽ�������Է�������������л���ķ���ʽ��
�ڡ����������ʾA������ֻ�С�C-C��������C-H�����͡�O-H�����������գ��ݴ˽���л���ķ���ʽ���ƶ�A���ܵĽṹ��ʽ��
��A�ĺ˴Ź�������ֻ���������շ壬�����к���3��Hԭ�ӣ��ݴ˽��A�ķ���ʽ��������ȷ��A�Ľṹ����д����ʽ��
����⣺��ͨ��Ũ��������3.6g��ˮ��������ˮ�����ʵ���Ϊ =0.2mol����n��H��=2×0.2mol=0.4mol��m��H��=0.4mol×1g/mol=0.4g����������������Һ����6.6�������ɶ�����̼��������������̼�����ʵ���Ϊ
=0.2mol����n��H��=2×0.2mol=0.4mol��m��H��=0.4mol×1g/mol=0.4g����������������Һ����6.6�������ɶ�����̼��������������̼�����ʵ���Ϊ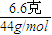 =0.15mol����n��C��=0.15mol��m��C��=0.15mol×12g/mol=1.8g�����л���A��m��O��=3.8g-0.4g-1.8g=1.6g����n��O��=
=0.15mol����n��C��=0.15mol��m��C��=0.15mol×12g/mol=1.8g�����л���A��m��O��=3.8g-0.4g-1.8g=1.6g����n��O��= =0.1mol������n��C����n��H����n��O��=0.15mol��0.4mol��0.1mol=3��8��2�����ʽΪC3H8O2�������Է���������֪���ʽ��Ϊ����ʽ��
=0.1mol������n��C����n��H����n��O��=0.15mol��0.4mol��0.1mol=3��8��2�����ʽΪC3H8O2�������Է���������֪���ʽ��Ϊ����ʽ��
�ʴ�Ϊ��C3H8O2��
�ں��������ʾA������ֻ�С�C-C��������C-H�����͡�O-H�����������գ�ͬһ��̼ԭ�������������ǻ����ȶ�
�ʷ��Ͻṹ�������л���A�Ľṹ��ʽΪ��CH3CH��0H��CH2OH CH2��OH��CH2CH2OH��
�ʴ�Ϊ��CH3CH��0H��CH2OH��CH2��OH��CH2CH2OH��
��A�ĺ˴Ź�������ֻ���������շ壬�����к���3��Hԭ�ӣ�����л���A�ĺ������֪��AΪCH2��OH��CH2CH2OH
��A�����������Ʒ�Ӧ�Ļ�ѧ����ʽΪCH2��OH��CH2CH2OH+2Na��NaOCH2CH2CH2ONa+H2����
A�����������ȣ�ͭ����������Ӧ�Ļ�ѧ����ʽ��CH2��OH��CH2CH2OH+O2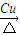 OHCCH2CHO+2H2O��
OHCCH2CHO+2H2O��
�ʴ�Ϊ��CH2��OH��CH2CH2OH+2Na��NaOCH2CH2CH2ONa+H2����
CH2��OH��CH2CH2OH+O2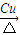 OHCCH2CHO+2H2O��
OHCCH2CHO+2H2O��
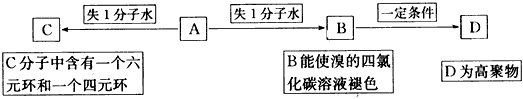
�����������л�����ƶϣ��Ѷ��еȣ�����ԭ���غ�ȷ���л���ķ���ʽ�ǽ���Ĺؼ�������ȼ�շ������л������ʽ��ԭ����
 ����ˮ��������̼�����ʵ������ٸ���ԭ���غ����Cԭ�ӡ���ԭ�ӵ����ʵ���������m=nM����Cԭ�ӡ���ԭ�ӵ����������������л�����Oԭ�ӵ�����������n=
����ˮ��������̼�����ʵ������ٸ���ԭ���غ����Cԭ�ӡ���ԭ�ӵ����ʵ���������m=nM����Cԭ�ӡ���ԭ�ӵ����������������л�����Oԭ�ӵ�����������n= ������ԭ�ӵ����ʵ�����ȷ�����ʽ�������Է�������������л���ķ���ʽ��
������ԭ�ӵ����ʵ�����ȷ�����ʽ�������Է�������������л���ķ���ʽ���ڡ����������ʾA������ֻ�С�C-C��������C-H�����͡�O-H�����������գ��ݴ˽���л���ķ���ʽ���ƶ�A���ܵĽṹ��ʽ��
��A�ĺ˴Ź�������ֻ���������շ壬�����к���3��Hԭ�ӣ��ݴ˽��A�ķ���ʽ��������ȷ��A�Ľṹ����д����ʽ��
����⣺��ͨ��Ũ��������3.6g��ˮ��������ˮ�����ʵ���Ϊ
 =0.2mol����n��H��=2×0.2mol=0.4mol��m��H��=0.4mol×1g/mol=0.4g����������������Һ����6.6�������ɶ�����̼��������������̼�����ʵ���Ϊ
=0.2mol����n��H��=2×0.2mol=0.4mol��m��H��=0.4mol×1g/mol=0.4g����������������Һ����6.6�������ɶ�����̼��������������̼�����ʵ���Ϊ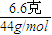 =0.15mol����n��C��=0.15mol��m��C��=0.15mol×12g/mol=1.8g�����л���A��m��O��=3.8g-0.4g-1.8g=1.6g����n��O��=
=0.15mol����n��C��=0.15mol��m��C��=0.15mol×12g/mol=1.8g�����л���A��m��O��=3.8g-0.4g-1.8g=1.6g����n��O��= =0.1mol������n��C����n��H����n��O��=0.15mol��0.4mol��0.1mol=3��8��2�����ʽΪC3H8O2�������Է���������֪���ʽ��Ϊ����ʽ��
=0.1mol������n��C����n��H����n��O��=0.15mol��0.4mol��0.1mol=3��8��2�����ʽΪC3H8O2�������Է���������֪���ʽ��Ϊ����ʽ���ʴ�Ϊ��C3H8O2��
�ں��������ʾA������ֻ�С�C-C��������C-H�����͡�O-H�����������գ�ͬһ��̼ԭ�������������ǻ����ȶ�
�ʷ��Ͻṹ�������л���A�Ľṹ��ʽΪ��CH3CH��0H��CH2OH CH2��OH��CH2CH2OH��
�ʴ�Ϊ��CH3CH��0H��CH2OH��CH2��OH��CH2CH2OH��
��A�ĺ˴Ź�������ֻ���������շ壬�����к���3��Hԭ�ӣ�����л���A�ĺ������֪��AΪCH2��OH��CH2CH2OH
��A�����������Ʒ�Ӧ�Ļ�ѧ����ʽΪCH2��OH��CH2CH2OH+2Na��NaOCH2CH2CH2ONa+H2����
A�����������ȣ�ͭ����������Ӧ�Ļ�ѧ����ʽ��CH2��OH��CH2CH2OH+O2
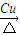 OHCCH2CHO+2H2O��
OHCCH2CHO+2H2O���ʴ�Ϊ��CH2��OH��CH2CH2OH+2Na��NaOCH2CH2CH2ONa+H2����
CH2��OH��CH2CH2OH+O2
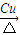 OHCCH2CHO+2H2O��
OHCCH2CHO+2H2O�������������л�����ƶϣ��Ѷ��еȣ�����ԭ���غ�ȷ���л���ķ���ʽ�ǽ���Ĺؼ�������ȼ�շ������л������ʽ��ԭ����

��ϰ��ϵ�д�
�����Ŀ
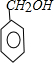
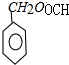 +H2O
+H2O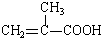
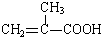 +HO-CH2CH2-OH
+HO-CH2CH2-OH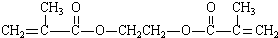 +2H2O
+2H2O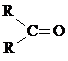
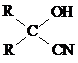
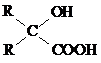
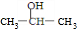 +O2
+O2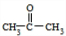 +2H2O��
+2H2O��