��Ŀ����
����Ŀ����25 �桢101 kPaʱ����Ӧ����ת����ϵ��ͼ��ʾ������˵������ȷ����

��֪��C(s)��O2(g)===CO��(g)������H1��CO(g)��![]() O2(g)===CO2(g) ��H2��
O2(g)===CO2(g) ��H2��
A. ��H2����282.9kJ��mol��1
B. 2C(s)��O2(g)===2CO(g)����H����221.2 kJ��mol��1
C. ��H1>��H2
D. CO2(g)�ֽ�ΪC(s)��O2(g)�����ȷ�Ӧ
���𰸡�C
��������
��������ת��ͼ��֪��C(s)��O2(g)===CO��(g)������H1 = -393.5 kJ��mol��1��CO(g)��1/2O2(g)===CO2(g) ��H2����282.9kJ��mol��1����ϸ�˹���ɼ���Ӧ���뻯ѧ��Ӧ����ʽ�Ĺ�ϵ����
��
A. ������������֪��COת��ΪCO2����Ӧ���ȣ����Ȼ�ѧ����ʽΪ��CO(g)��1/2O2(g)===CO2(g) ��H2����282.9kJ��mol��1��A����ȷ��
B. Cת��ΪCO2�ų����������Ȼ�ѧ����ʽΪ��C(s)��O2(g)===CO��(g)������H1 = -393.5 kJ��mol��1����ϸ�˹���ɿ�֪��Cת��ΪCO���Ȼ�ѧ����ʽΪ��2C(s)��O2(g)===2CO(g)����H������H1 kJ��mol��1-��H2����2��[-393.5 kJ��mol��1-��-282.9 kJ��mol��1��]��2 = ��221.2 kJ��mol��1��B����ȷ��
C. ��H1 = -393.5 kJ��mol��1����H2����282.9kJ��mol��1������H1<��H2��C�����
D. C(s)��O2(g)��ӦΪ���ȷ�Ӧ����CO2(g)�ֽ�ΪC(s)��O2(g)�����ȷ�Ӧ��D����ȷ��
��ѡC��

����Ŀ��һ�����������������ȵ��������dz����Ĵ�����Ⱦ�Ȼ�������ڹ�ҵ�����Ź㷺����;��
��֪���������£�2NO��Na2O2=2NaNO2�������������£�NO��NO2��������MnO4����Ӧ����NO3����
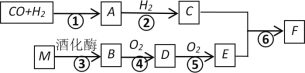
��.��ľ̿��Ũ���ᡢˮ��ͭΪԭ�����ɵ�һ��������������Ʒ�Ӧ�Ʊ��������Ƶ�װ������ͼ��ʾ��
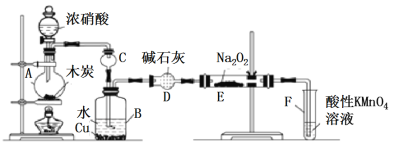
(1)A�з�Ӧ�Ļ�ѧ����ʽ_____________��
(2)װ��B�й۲쵽����Ҫ����______________��
(3)����D��������___________________��
(4)A�е���Ũ����֮ǰ��Ӧ��ͨ��N2һ��ʱ�䣬ԭ����____________��
(5)д��NO������KMnO4���������ӷ�Ӧ����ʽ______________��
(6)����װ��D����E�в����NaNO2�⣬����_______(�ѧʽ)��
��.ij�о���ѧϰС���NO2��Na2O2�ܷ�����Ӧ����̽����
����1���������
����һ��NO2��Na2O2����Ӧ��
�������NO2�ܱ�Na2O2������
��������______________________________________________��
����2�����ʵ��
(1)ʵ��ʱ����NO2����ͨ��ʢ��Na2O2�IJ������У�����ɫ��ĩ��ȫ��ɰ�ɫ��˵������________��������
(2)Ϊ��֤������Ƿ��������С��ͬѧ�������¶���ʵ���о���������±����ݡ�
ʵ�鲽��(��Ҫ��д����������̣��Լ���������ѡ) | Ԥ�ڵ�ʵ�������� |
ȡ�����İ�ɫ���������Թ��У�������ˮ�ܽ⣬ ��__________________________________________ | ��___________________________ |
����Ŀ����ȥ�������������������ʡ�ѡ�õ��Լ���ȷ����
ѡ�� | ����(����) | �Լ� |
A | Al2O3(SiO2) | ����NaOH��Һ |
B | CO2(HCl) | ����Na2CO3��Һ |
C | Al2O3(Fe2O3) | Al��/���� |
D | Na2O2(Na2O) | O2/���� |
A. A B. B C. C D. D