��Ŀ����
����Ŀ�������й�˵����ȷ����
A. һ�������·�ӦN2+3H2![]() 2NH3���ﵽƽ��ʱ��3v��(H2)=2v��(NH3)
2NH3���ﵽƽ��ʱ��3v��(H2)=2v��(NH3)
B. 25��ʱ��0.1 mol/L��NaHB��Һ�����ԣ�˵��H2BΪǿ��
C. ��pH=a+1�İ�ˮϡ��ΪpH=a�Ĺ����У�c(OH��)/c(NH3H2O)��С
D. 10mLŨ��Ϊ1mol/L�������������Zn�۷�Ӧ��������������CH3COONa��Һ�����ܽ��ͷ�Ӧ���ʣ��ֲ�Ӱ��H2������
���𰸡�D
��������
A.�����Ƿ�ﵽƽ�⣬����ͬһ��������ʱ�ʼ�յ��ڻ�ѧ����������![]() =
=![]() ����A����
����A����
B.25��ʱ��0.1 mol/L��NaHB��Һ���������������ֿ��ܣ�1��H2BΪǿ����NaHB=Na++H++B2-ʹ��Һ����������2��H2BΪ������NaHB��Һ��HB-�ĵ���̶ȴ���ˮ��̶ȣ�ʹ��Һ�����ԣ���B����
C.��ˮϡ�Ͱ�ˮ������ƽ�������ƶ���n(OH��)������n(NH3H2O)��С������c(OH��)/c(NH3H2O)= n(OH��)/ n(NH3H2O)����C������
D.CH3COONa�����ᷴӦ���ɴ��ᣬ���C��H+����С�����ͷ�Ӧ�������������ṩ��H+�����ʵ������䣬���Բ�Ӱ��H2����������D��ȷ��
��ȷ����D��

����Ŀ��H2S�ڽ������ӵļ���������ú��������������ҪӦ�á���ش�
��.��ҵ��һ���Ʊ�H2S�ķ������ڴ��������������£�����Ȼ����SO2��Ӧ��ͬʱ���������ܲ������ѭ���������
(1)�÷�Ӧ�Ļ�ѧ����ʽΪ_____________��
��.H2S�����ڼ��ͳ������������ӡ�
(2)H2S�ĵ�һ�����뷽��ʽΪ________��
(3)��֪��25 ��ʱ��Ksp(SnS)��1.0��10��25��Ksp(CdS)��8.0��10��27�����¶��£���Ũ�Ⱦ�Ϊ0.1 mol��L��1��CdCl2��SnCl2�Ļ����Һ��ͨ��H2S����Sn2����ʼ����ʱ����Һ��c(Cd2��)��________(��Һ����仯���Բ���)��
��.H2S��ú����ԭ����������̵���Ҫ�м��塣��Ӧԭ��Ϊ
��.COS(g)��H2(g) ![]() H2S(g)��CO(g)����H����7 kJ��mol��1��
H2S(g)��CO(g)����H����7 kJ��mol��1��
��.CO(g)��H2O(g) ![]() CO2(g)��H2(g)����H����42 kJ��mol��1��
CO2(g)��H2(g)����H����42 kJ��mol��1��
(4)��֪������1 mol�����еĻ�ѧ���������յ����������ʾ��
���� | COS(g) | H2(g) | CO(g) | H2S(g) | H2O(g) | CO2(g) |
����/(kJ��mol��1) | 1 319 | 442 | x | 678 | 930 | 1 606 |
����x��________��
(5)��10 L�ݻ�������ܱ������г���1 mol COS(g)��1 mol H2(g)��1 mol H2O(g)����������������Ӧ��������������ʱ����ϵ��CO��ƽ������������¶�(T)�Ĺ�ϵ��ͼ��ʾ��
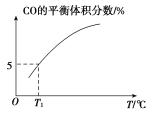
�������¶����ߣ�CO��ƽ���������_____(����������������С��)��ԭ��Ϊ_______
��T1��ʱ�����ƽ��ʱ��ϵ��COS�����ʵ���Ϊ0.80 mol������¶��£�COS��ƽ��ת����Ϊ_____����Ӧ����ƽ�ⳣ��Ϊ_____(������λ��Ч����)��
����Ŀ��̼�͵��Ļ����������������й㷺���ڡ��ش��������⣺
(1)��ת������ʹ����β���е���Ҫ��Ⱦ�CO��NOx��̼�⻯����������Ӧ�����������ʣ���������β����Ⱦ��
��֪��N2(g)+O2(g)=2NO(g) ��H1=+180.5 kJ/mol��
2C(s)+O2(g)=2CO(g) ��H2=��221.0 kJ/mol��
C(s)+O2(g)=CO2(g) ��H3=��393.5 kJ/mol
��β��ת����Ӧ2NO(g) +2CO(g)=N2(g)+2CO2(g)����H=___________��
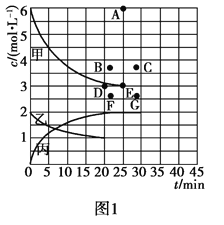
(2)����һ����Ҫ�Ļ���ԭ�ϣ��ڹ�ũҵ�������й㷺��Ӧ�á���773 Kʱ���ֱ�2.00 mol N2��6.00 mol H2����һ���̶��ݻ�Ϊ1 L���ܱ������У����ŷ�Ӧ�Ľ��У�����������n(H2)��n(NH3)�뷴Ӧʱ��(t)�Ĺ�ϵ�����ʾ��
t/min | 0 | 5 | 10 | 15 | 20 | 25 | 30 |
n(H2)/mol | 6.00 | 4.50 | 3.60 | 3.30 | 3.03 | 3.00 | 3.00 |
n(NH3)/mol | 0 | 1.00 | 1.60 | 1.80 | 1.98 | 2.00 | 2.00 |
�ٸ��¶��£�����ͬ�ݻ�����һ������Ͷ��N2��H2��NH3����Ũ�Ⱦ�Ϊ3 mol/L�����ʱv��____v��(������������С��������������)��
���ɱ��е�ʵ�����ݿɵõ���c��t���Ĺ�ϵ����ͼ1��ʾ����ʾc(N2)��t��������______���ڴ��¶��£�����ʼ����4 mol N2��12 mol H2����Ӧ�մﵽƽ��ʱ����ʾc(H2)��t����������Ӧ�ĵ�Ϊ________��
![]() N2(g)+CO2(g) ��H=��34.0kJ/mol���û���̿��NO������������֪���ܱ������м���������C��һ������NO���壬���ֺ�ѹ���NO��ת�������¶ȵı仯��ͼ2��ʾ��
N2(g)+CO2(g) ��H=��34.0kJ/mol���û���̿��NO������������֪���ܱ������м���������C��һ������NO���壬���ֺ�ѹ���NO��ת�������¶ȵı仯��ͼ2��ʾ��
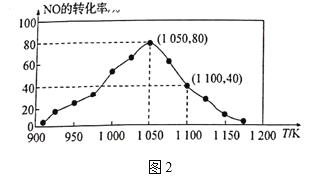
����ͼ��֪��1050Kǰ��Ӧ��NO��ת�������¶����{��������ԭ��Ϊ_______����1100Kʱ��CO2���������Ϊ__________��
����ij���ʵ�ƽ���ѹ���������ʵ���Ũ��Ҳ���Ա�ʾ��ѧƽ�ⳣ��(����Kp)����1050K��1.1��106Paʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��Kp=______[��֪�������ѹ(P��)=������ѹ(Pa)���������]��
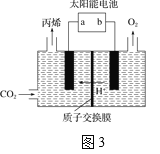
(4)�����Ե������Һ�У��Զ��Բ������缫����CO2ת��Ϊ��ϩ��ԭ����ͼ3��ʾ
��̫���ܵ�صĸ�����_______(����a������b��)
�����ɱ�ϩ�ĵ缫��Ӧʽ��___________��