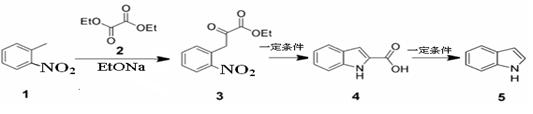
��Ŀ����
��֪��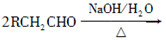
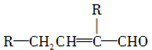
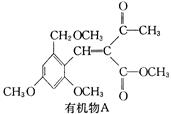
ˮ����EΪ���������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�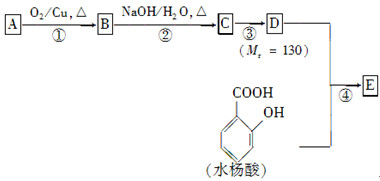
��ش��������⣺
(1)һԪ��A��������������ԼΪ21.6%����A�ķ���ʽΪ________���ṹ������ʾAֻ��һ������A������Ϊ________��
(2)B�������Ƶ�Cu(OH)2������Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ__________________________��
(3)C��________�ֽṹ����һ��ȡ��������C�����������ţ���ʹ�õ��Ⱥ�˳��д�������Լ���________��
(4)�ڢ۲��ķ�Ӧ����Ϊ________��D���������ŵ�����Ϊ________��
(5)д��ͬʱ��������������ˮ��������ͬ���칹��Ľṹ��ʽ��__________________________��
a����������6��̼ԭ����һ��ֱ���ϣ�
b�����������������Ű���ˮ������еĹ����š�
(6)�ڢܲ��ķ�Ӧ����Ϊ________��д��E�Ľṹ��ʽ��______________��
(1)C4H10O��1������(��������)
(2)CH3CH2CH2CHO��2Cu(OH)2��NaOH CH3CH2CH2COONa��Cu2O����3H2O
CH3CH2CH2COONa��Cu2O����3H2O
(3)2��������Һ��ϡ���ᡢ��ˮ(������������)
(4)��ԭ��Ӧ(��ӳɷ�Ӧ)���ǻ�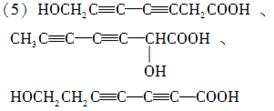
(6)ŨH2SO4������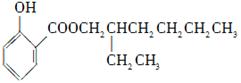
����

 ����ȫ���ִʾ��ƪ��ϵ�д�
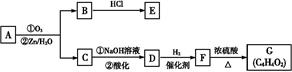
����ȫ���ִʾ��ƪ��ϵ�д�ij�������Ա�Ϊ��Ҫԭ�ϣ���������·�ߺϳ�����ҩ����������

�ش��������⣺
��1�����������ӣ�����˵����ȷ����________��
| A��1 mol�����������Ժ�2 mol NaOH��Ӧ |
| B��������������Ӧ |
| C���ɷ���ˮ�ⷴӦ |
| D�������巢��ȡ����Ӧ |
��3��д��B��C�Ļ�ѧ����ʽ__________________________________________��
��4��д��������F�Ľṹ��ʽ__________��
��5��д��ͬʱ��������������F��ͬ���칹��Ľṹ��ʽ________________________________________________________��д��3�֣���
�����������ұ����������ֲ�ͬ��ѧ��������ԭ�ӡ�
���ܷ���������Ӧ��
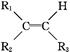
��6���Ա�����ϩΪԭ�Ͽɺϳɾ۱���ϩ������ƺϳ�·�ߣ����Լ����ܼ���ѡ��
ע���ϳ�·�ߵ���д��ʽ��������ʾ������ͼ��
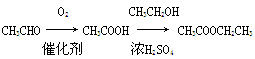




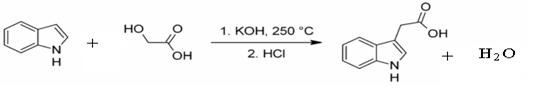
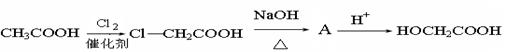
 ����д�����������ᷢ����Ӧ�Ļ�ѧ����ʽ������д��Ӧ�������� ��
����д�����������ᷢ����Ӧ�Ļ�ѧ����ʽ������д��Ӧ�������� ��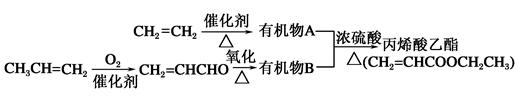
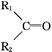 +
+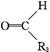
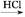 R��OH(RΪ֬����������������)
R��OH(RΪ֬����������������)


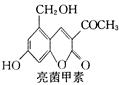
 ���ʼ��ԣ�
���ʼ��ԣ�




