题目内容
(1)(2分)下列有关实验操作或判断不正确的是 ________(填序号,多选扣分)。
E.在天平左右两盘中各放一张白纸后,即可将NaOH固体放在白纸上称量
(2) (2分)双氧水(H2O2)是极弱的电解质, H2O2溶液显弱酸性。若把H2O2看成二元弱酸,请写出它在水中的电离方程式 ,
(3) (4分)由氢气和氧气反应生成1mol水蒸气,放热241.8kJ,写出该反应的热化学方程式 。若1g水蒸气转化成液态水放热2.444 kJ,则H2的燃烧热ΔH= kJ·mol-1
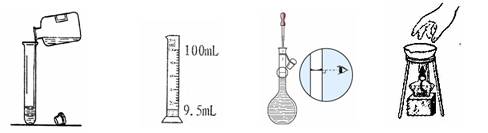
| A.配制一定物质的量浓度溶液,定容时俯视刻度线会导致所配溶液浓度偏小 |
| B.少量浓硫酸沾在皮肤上,立即用氢氧化钠溶液冲洗 |
| C.液溴有毒且易挥发,需盛放在磨口的细口瓶里,并水封保存 |
| D.100 mL容量瓶可用于配制95 mL 0.1 mol/L NaCl溶液 |
(2) (2分)双氧水(H2O2)是极弱的电解质, H2O2溶液显弱酸性。若把H2O2看成二元弱酸,请写出它在水中的电离方程式 ,
(3) (4分)由氢气和氧气反应生成1mol水蒸气,放热241.8kJ,写出该反应的热化学方程式 。若1g水蒸气转化成液态水放热2.444 kJ,则H2的燃烧热ΔH= kJ·mol-1
(8分)ABE (2分) H2O2 H++HO2- HO2-
H++HO2- HO2-  H++O22-(2分)
H++O22-(2分)
2H2(g)+O2(g)="2H2O(g) " ΔH="-483.6" kJ·mol-1 (2分) -285.8.(2分)
 H++HO2- HO2-
H++HO2- HO2-  H++O22-(2分)
H++O22-(2分)2H2(g)+O2(g)="2H2O(g) " ΔH="-483.6" kJ·mol-1 (2分) -285.8.(2分)
略

练习册系列答案
相关题目


 )简述该实验的主要实验步骤
)简述该实验的主要实验步骤  低于刻度线,又滴加蒸馏水至刻度。对所配溶液浓度的影响 。
低于刻度线,又滴加蒸馏水至刻度。对所配溶液浓度的影响 。