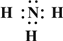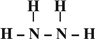��Ŀ����
��֪������Ԫ�ص�ԭ�������Ĵ�С˳��ΪC��A��B��D��E��A��Cͬ���ڣ�B��Cͬ���壻A��B�γɵ����ӻ�����A2B�����е����ӵĵ���������ͬ�����������Ϊ30��D��E���γ�4��10���ӷ��ӡ��Իش��������⣺
1. д������Ԫ�ص����ƣ�A ��B
2. д��DԪ�ص�ԭ�ӽṹʾ��ͼ ��CԪ�������ڱ��е�λ�� ��
3. д���������ʵĵ���ʽ
E��B�γɵĻ����
A��B��E�γɵĻ����
D��E�γɵĻ����
4. д��C������������Ӧˮ�����Ũ��Һ��ͭ��Ӧ�Ļ�ѧ����ʽ
д��D������������Ӧˮ�����ϡ��Һ��ͭ��Ӧ�����ӷ���ʽ
�����11�֣�ǰ3С��ÿ�������1�֣���4С���������ʽ2�֣�
1��A
�� ��B �� 2��  ��
�������ڢ�A��
��
�������ڢ�A��
3�� ( ��
( �� ) ��
) ��  ��
�� 
4�� ��
��
����ע��Ũ����д������������ƽ�������֣�
��������
�����������1��A��B�γɵ����ӻ�����A2B�����е����ӵĵ���������ͬ�����������Ϊ30������A��Na��B��O������Ϊԭ�������Ĵ�С˳��ΪC��A��B��D��E��A��Cͬ���ڣ�B��Cͬ���壬����C��S��D��E���γ�4��10���ӷ��ӣ����D��N��E��H��
��2����Ԫ�ص�ԭ��������7����ԭ�ӽṹʾ��ͼΪ ��S��ԭ��������16��λ�ڵ������ڢ�A�塣
��S��ԭ��������16��λ�ڵ������ڢ�A�塣
��3��H��O�����γ�ˮ��˫��ˮ�����ǹ��ۻ���������ʽ�ֱ��� ��
�� ��A��B��E�γɵĻ��������������ƣ��������Ӽ��ͼ��Լ�������ʽ��
��A��B��E�γɵĻ��������������ƣ��������Ӽ��ͼ��Լ�������ʽ�� ��D��E�γɵĻ������ǰ��������м��Լ�������ʽ��
��D��E�γɵĻ������ǰ��������м��Լ�������ʽ�� ��
��
��4��C������������Ӧˮ���������ᣬŨ������������ԣ���ͭ��Ӧ�ķ���ʽ��2H2SO4(Ũ)��Cu CuSO4��2H2O��SO2����D������������Ӧˮ���������ᣬ����Ҳ���������ԣ���ͭ��Ӧ�����ӷ���ʽ��3Cu��8H����2NO3��=3Cu2����4H2O��2NO����
CuSO4��2H2O��SO2����D������������Ӧˮ���������ᣬ����Ҳ���������ԣ���ͭ��Ӧ�����ӷ���ʽ��3Cu��8H����2NO3��=3Cu2����4H2O��2NO����
���㣺����Ԫ�����ڱ��Ľṹ�͵���ʽ������ʽ���й���д
��������������λ�������Ե��ۺϿ��飬��Ҫ��������Ԫ�����ڱ��Ľṹ��Ȼ������֪��������һ�ƶϼ��ɡ�

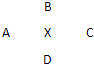 ��A��B��C��D����Ԫ�������ڱ��зֱ���Ԫ��X�����ܣ���ͼ������֪XԪ�����������Ļ�ѧʽΪX2O5��������Ԫ������һ��Ԫ�ص�ԭ�Ӱ뾶������������ͬ������С�ģ���ȷ����
��A��B��C��D����Ԫ�������ڱ��зֱ���Ԫ��X�����ܣ���ͼ������֪XԪ�����������Ļ�ѧʽΪX2O5��������Ԫ������һ��Ԫ�ص�ԭ�Ӱ뾶������������ͬ������С�ģ���ȷ����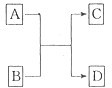 ��2013?����ģ�⣩��һ��X��Y��Z��L��M����Ԫ�ص�ԭ��������������X����������Y���ڲ��������ȣ�Yԭ�ӵ������������Ǵ�����������������Z��L�ǿ����к������Ķ���Ԫ�أ�M�ǵؿ��к�����ߵĽ���Ԫ�أ��ش��������⣺
��2013?����ģ�⣩��һ��X��Y��Z��L��M����Ԫ�ص�ԭ��������������X����������Y���ڲ��������ȣ�Yԭ�ӵ������������Ǵ�����������������Z��L�ǿ����к������Ķ���Ԫ�أ�M�ǵؿ��к�����ߵĽ���Ԫ�أ��ش��������⣺ ��
��