��Ŀ����
����Ŀ����ʯ�ͻ�����Ʒ1,3-����ϩ�ϳɻ����м��ƷD ��![]() ���ĺϳ�·�����£�
���ĺϳ�·�����£�
��֪��

��1������D����������������Ϊ_________��һ�������£�1 molD�����_________molH2�����ӳɷ�Ӧ��
��2��д���ڢķ�Ӧ���ͷֱ���_________��_________��
��3���ܷ�Ӧ�Ļ�ѧ����ʽ_________���ݷ�Ӧ�Ļ�ѧ����ʽ_________��
��4��A ��ϵͳ������_________��
��5��д��������D��Ϊͬ���칹�������������������л���ṹ��ʽ_________��
�� ����FeCl3��Һ������ɫ��Ӧ
�� �ܷ���������Ӧ
�� �˴Ź���������ʾ��5���
��6������ƺ���������![]() �ϳ�
�ϳ�![]() ��������ԭ����ѡ���÷�Ӧ����ͼ��ʾ����ע����Ҫ�ķ�Ӧ��������_______
��������ԭ����ѡ���÷�Ӧ����ͼ��ʾ����ע����Ҫ�ķ�Ӧ��������_______
���𰸡� ̼̼˫�� ȩ�� 4 �ӳɷ�Ӧ����ԭ��Ӧ�� ��ȥ��Ӧ CH2(OH)CH2CH2CH2OH+O2 ![]() OHCCH2CH2CHO+2H2O 2OHCCH2CH2CHO��
OHCCH2CH2CHO+2H2O 2OHCCH2CH2CHO��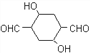 1��4-������
1��4-������ 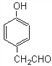
![]()
����������Ӧ��Ϊ±������ˮ�ⷴӦɾ������AΪHOCH2CH2CH2CH2OH��A����������ȩ��BΪOHCCH2CH2CHO��B�ڼ��������·�����Ӧ����C��CΪ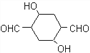 ������D�Ľṹ��C�����ǻ�����ȥ��Ӧ����D��
������D�Ľṹ��C�����ǻ�����ȥ��Ӧ����D��
(1)D Ϊ![]() ������������������̼̼˫����ȩ����һ�������£�ȩ����̼̼˫�������������ӳɣ�1 molD�����4molH2�����ӳɷ�Ӧ���ʴ�Ϊ��̼̼˫����ȩ����4��
������������������̼̼˫����ȩ����һ�������£�ȩ����̼̼˫�������������ӳɣ�1 molD�����4molH2�����ӳɷ�Ӧ���ʴ�Ϊ��̼̼˫����ȩ����4��
(2)��Ӧ��Ϊ̼̼˫���ļӳɷ�Ӧ����Ӧ��Ϊ ��ȥ��Ӧ���ʴ�Ϊ���ӳɷ�Ӧ����ȥ��Ӧ��
��ȥ��Ӧ���ʴ�Ϊ���ӳɷ�Ӧ����ȥ��Ӧ��
(3)��Ӧ��Ϊ���Ĵ���������Ӧ�Ļ�ѧ����ʽΪCH2(OH)CH2CH2CH2OH+O2 ![]() OHCCH2CH2CHO+2H2O����Ӧ�ݵĻ�ѧ����ʽΪ2OHCCH2CH2CHO��
OHCCH2CH2CHO+2H2O����Ӧ�ݵĻ�ѧ����ʽΪ2OHCCH2CH2CHO�� ���ʴ�Ϊ��CH2(OH)CH2CH2CH2OH+O2
���ʴ�Ϊ��CH2(OH)CH2CH2CH2OH+O2 ![]() OHCCH2CH2CHO+2H2O��2OHCCH2CH2CHO��
OHCCH2CH2CHO+2H2O��2OHCCH2CH2CHO��  ��
��
(4)AΪHOCH2CH2CH2CH2OH��ϵͳ����Ϊ1��4-���������ʴ�Ϊ��1��4-��������
(5)D Ϊ![]() ���� ����FeCl3��Һ������ɫ��Ӧ��˵�����з��ǻ����� �ܷ���������Ӧ��˵������ȩ������ �˴Ź���������ʾ��5��壬�����������л���Ϊ
���� ����FeCl3��Һ������ɫ��Ӧ��˵�����з��ǻ����� �ܷ���������Ӧ��˵������ȩ������ �˴Ź���������ʾ��5��壬�����������л���Ϊ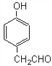 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
(6)��![]() �ϳ�
�ϳ�![]() ���������Ƚ�
���������Ƚ�![]() ����Ϊ���ᣬ���������ɣ��ϳ�·��Ϊ
����Ϊ���ᣬ���������ɣ��ϳ�·��Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��

����Ŀ����úΪԭ�Ͽɺϳ�һϵ��ȼ�ϡ�
��1����֪����2H2��g��+O2��g��= 2H2O��g����H=��483.6kJ/mol
��CH3OH(g)+H2O(g)=CO2(g)+3H2(g)��H=��49.0kJ/mol
��д���״�ȼ������H2O��g�����Ȼ�ѧ����ʽ_________;
��2����1L�ܱ������м���2mol CO��4mol H2�����ʵ��Ĵ��������£�������Ӧ��2CO��g��+4H2��g��![]() CH3OCH3��l��+H2O��l����H=+71kJ/mol
CH3OCH3��l��+H2O��l����H=+71kJ/mol
�ٸ÷�Ӧ�ܷ�_________�Է����У���ܡ��������ܡ������жϡ���
������������˵���˷�Ӧ�ﵽƽ��״̬����_________��
a����������ƽ����Է����������ֲ���
b��CO��H2��ת�������
c��CO��H2������������ֲ���
d�����������ܶȱ��ֲ���
e��1mol CO���ɵ�ͬʱ��1mol O��H������
��3��CO2��g��+3H2��g��![]() CH3OH��g��+H2O��g����H��0��һ�������£�ij��Ӧ�����в����������±���
CH3OH��g��+H2O��g����H��0��һ�������£�ij��Ӧ�����в����������±���
��Ӧ���� | ��Ӧʱ�� | CO2(mol) | H2(mol) | CH3OH(mol) | H2O(mol) |
���� ���� (T1�桢 2L) | 0min | 2 | 6 | 0 | 0 |
10min | 4.5 | ||||
20min | 1 | ||||
30min | 1 |
��0��10min�ڣ���H2O��g����ʾ�Ļ�ѧ��Ӧ����v(H20)=_________mol/(L��min)
�ڴﵽƽ��ʱ���÷�Ӧ��ƽ�ⳣ��K=_________���÷�����ʾ����ƽ��ʱH2��ת������_________��
���������������������£���30minʱ�ı��¶�ΪT
��4���ü��ѣ�CH3OCH3����Ϊȼ�ϵ�ص�ԭ��,��д���ڼ��Խ����е�ظ�����Ӧʽ_________��
����Ŀ���ȱ���Ⱦ�ϡ�ҽҩ��ҵ���й㷺��Ӧ�ã�ijʵ��С����������װ�úϳ��ȱ���֧���õ�����̨����ʡ�ԣ���ͨ��һ�������ᴿ�ȱ���
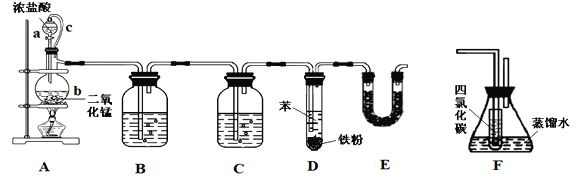
��Ӧ��Ͳ������������б����£�
�ܶ�/g��cm��3 | �е�/�� | ˮ���ܽ��� | |
�� | 0.879 | 80.1 | �� |
�ȱ� | 1.11 | 131.7 | ���� |
�밴Ҫ��ش��������⡣
��1��װ��A����c��������______________��װ��E��������__________________��
��2��ʵ��ʱ��ʹa�е�Ũ���Ỻ�����£��ɹ۲쵽����b�ڵ�������________________��д����Ӧ�����ӷ���ʽ______________________________________��
��3��Ϊ֤�������ͱ���������ȡ�������Ǽӳɷ�Ӧ����С����װ��F˵������װ��F����________֮�䣨����ĸ����F��С�Թ���CCl4��������___________________������ʹ�õ��Լ���______________��
��4����֪D�м���5 mL���������ᴿ���ռ����ȱ�3.0 g�����ȱ��IJ���Ϊ_________%��������λ��Ч���֣���





