��Ŀ����
����3�������ȵ��ܱ����������������з�Ӧ��CO2(g) + H2(g) H2O(g) + CO(g)��
H2O(g) + CO(g)��
��Ӧ�������¶���ͬ������ʼŨ�Ȳ�ͬ������ �ף�n(CO2) �� n(H2) �� 1 mol��
��:n(CO2)��1 mol,n(H2)��2mol�� ��:n(CO2)��n(H2)��1mol,n[H2,O(g)]��1 mol��
�ﵽƽ��ʱCO�����ʵ����ɴ�С��˳���� �� ��
 H2O(g) + CO(g)��
H2O(g) + CO(g)����Ӧ�������¶���ͬ������ʼŨ�Ȳ�ͬ������ �ף�n(CO2) �� n(H2) �� 1 mol��
��:n(CO2)��1 mol,n(H2)��2mol�� ��:n(CO2)��n(H2)��1mol,n[H2,O(g)]��1 mol��
�ﵽƽ��ʱCO�����ʵ����ɴ�С��˳���� �� ��
| A���ң��ף��� | B���ף������� | C���ң������� | D���ף��ң��� |
A
��

��ϰ��ϵ�д�
 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�
�����Ŀ
 2NH3(g) ��H����92.4kJ��mol��1����ش�
2NH3(g) ��H����92.4kJ��mol��1����ش� H2��NH3����������Ӧ������ʱ��Ĺ�ϵ����ͼ��ʾ��
H2��NH3����������Ӧ������ʱ��Ĺ�ϵ����ͼ��ʾ��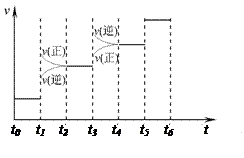

 2SO3��g�������ﵽƽ�⡣��������У�����������������䣬����������ѹǿ���䣬����������SO2��ת����Ϊp%������������SO2��ת���ʣ�������
2SO3��g�������ﵽƽ�⡣��������У�����������������䣬����������ѹǿ���䣬����������SO2��ת����Ϊp%������������SO2��ת���ʣ������� g��+B��g��
g��+B��g��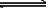 nC��g����H=Q���ڲ�ͬ�����·�Ӧ�������C�İٷֺ�
nC��g����H=Q���ڲ�ͬ�����·�Ӧ�������C�İٷֺ� ���ͷ�Ӧ������ʱ��Ĺ�ϵ���ߡ������й�������һ����ȷ���ǣ���
���ͷ�Ӧ������ʱ��Ĺ�ϵ���ߡ������й�������һ����ȷ���ǣ���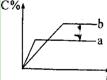
 2CO(g) ��
2CO(g) �� �����жϷ�Ӧ�Ѿ��ﵽ��ѧƽ��״̬����
�����жϷ�Ӧ�Ѿ��ﵽ��ѧƽ��״̬���� qZ��g����H;��Ӧ��Y�뷴Ӧʱ��t���¶�T��ѹǿp�Ĺ�ϵ������ͼ����ͨ���Դ����ߵķ������ж�������������ȷ����
qZ��g����H;��Ӧ��Y�뷴Ӧʱ��t���¶�T��ѹǿp�Ĺ�ϵ������ͼ����ͨ���Դ����ߵķ������ж�������������ȷ����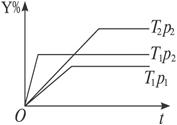
 2Z(g) �˷�Ӧ�ﵽƽ��ı�־��
2Z(g) �˷�Ӧ�ﵽƽ��ı�־�� Ag���̣���Fe3��������Ӧ���ȣ�
Ag���̣���Fe3��������Ӧ���ȣ� 2NO2�CQ��Q>0������N2O4��NO2Ũ�ȱ仯��ͼ��ʾ��
2NO2�CQ��Q>0������N2O4��NO2Ũ�ȱ仯��ͼ��ʾ��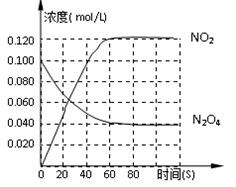
 0s��ƽ�����淴Ӧ�����ƶ������ܵ�ԭ���ǣ� ��
0s��ƽ�����淴Ӧ�����ƶ������ܵ�ԭ���ǣ� ��