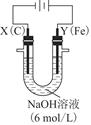
��Ŀ����
��֪Ǧ���صĹ���ԭ��ΪPb+PbO2+2H2SO4  2PbSO4+2H2O��������ͼװ�ý��е��(���Һ����)����õ�Ǧ������ת��0.4 mol ����ʱ���缫����������11.2 g����ش��������⡣
2PbSO4+2H2O��������ͼװ�ý��е��(���Һ����)����õ�Ǧ������ת��0.4 mol ����ʱ���缫����������11.2 g����ش��������⡣
(1)A��Ǧ���ص� ����Ǧ����������ӦʽΪ ���ŵ�����е��Һ���ܶ� (���С�����������䡱)��
(2)Ag�缫�ĵ缫��Ӧʽ�� ���õ缫�ĵ缫���ﹲ g��
(3)Cu�缫�ĵ缫��Ӧʽ�� ��CuSO4��Һ��Ũ�� (���С�����������䡱)��
(4)��ͼ��ʾ�����й�����ij����(������x)��ʱ��ı仯���ߣ���x��ʾ ��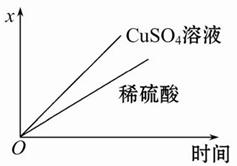
a.��U�ι��в�������������
b.��U�ι������������ļ�����
c.��U�������������������
(1)�� PbO2+4H++SO42-+2e-=PbSO4+2H2O ��С
(2)2H++2e-=H2�� 0.4
(3)Cu-2e-=Cu2+ ���� (4)b
����

 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д���1����ͨп�̸ɵ�صĽṹ��ͼ��ʾ���ش��������⡣
�ٵ���е������ҺΪ________��
��������ӦʽΪ________��
�۷ŵ�ʱ��NH4+��________������������������ƶ���
��2���ϵ���е�пƤ������ʵ��������������пƤ�ʹ�п���ֱ���ͬŨ�ȵ�ϡ���ᷴӦ�������������ʽϴ����________��ԭ����________________________��
���ù����Ĵ�п����һ������ϡ���ᷴӦ��Ϊ�˼ӿ췴Ӧ�����ֲ�Ӱ������������������д�ʩ���е���________������ţ���
| A���� |
| B��������������ͭ |
| C��������������ͭ��Һ |
| D����ˮ |