ƒøƒ⁄»ð
°æƒø°ø¢Ò.ø∆—ߺ“¿˚”√ô—ÙƒÐ∑÷Ω‚ÀÆ…˙≥…µƒ«‚∆¯‘⁄¥þªØº¡◊˜”√œ¬”Î∂˛—ıªØú∑¥”¶…˙≥…º◊¥º£¨≤¢ø™∑¢≥ˆ÷±Ω”“‘º◊¥ºŒ™»º¡œµƒ»º¡œµÁ≥ÿ°£º∫÷™H2(g)°¢CO(g)∫ÕCH3OH(l)µƒ»º…’»»¶§H∑÷±Œ™-285.8kJ°§mol-1°¢-283.0 kJ°§mol-1 ∫Õ-726.5 kJ°§mol-1°£«Îªÿ¥œ¬¡–Œ £∫
£®1£©”√ô—ÙƒÐ∑÷Ω‚10molÀÆœ˚∫ƒµƒƒÐ¡ø «______________kJ°£
£®2£©º◊¥º≤ªÕÍ»´»º…’…˙≥…“ª—ıªØú∫Õ“∫èÀƵƒ»»ªØ—ß∑Ω≥Ã ΩŒ™______________________________°£
£®3£©‘⁄»ðª˝Œ™2Lµƒ√б’»ð∆˜÷–£¨”…CO2∫ÕH2∫œ≥…º◊¥º£¨±£≥÷∆‰À˚Ãıº˛≤ª±‰£¨ÃΩæøŒ¬∂»∂‘∑¥”¶µƒ”∞œÏ£¨ µ—ÈΩ·π˚»Áœ¬ÕºÀ˘ æ(T1°¢T2æ˘¥Û”⁄300°Ê)°£œ¬¡–Àµ∑®’˝»∑µƒ «________________£®ÃÓ–Ú∫≈£©
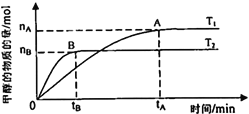
¢ŸŒ¬∂»Œ™T1 ±£¨¥”∑¥”¶ø™ ºµΩ∆Ω∫‚£¨…˙≥…º◊¥ºµƒ∆Ωæ˘ÀŸ¬ Œ™v(CH3OH) =![]() mol°§L-1°§min-1
mol°§L-1°§min-1
¢⁄∏√∑¥”¶‘⁄T1 ±µƒ∆Ω∫‚≥£ ˝±»T2 ±µƒ–°
¢€∏√∑¥”¶Œ™∑≈»»∑¥”¶
¢Ð¥¶”⁄Aµ„µƒ∑¥”¶ÃÂœµ¥”T1±‰µΩT2£¨¥ÔµΩ∆Ω∫‚ ±![]() ‘ˆ¥Û
‘ˆ¥Û
£®4£©‘⁄T1Œ¬∂» ±£¨Ω´1mol CO2∫Õ3mol H2≥‰»Î“ª√б’∫„»ð»ð∆˜÷–£¨≥‰∑÷∑¥”¶¥ÔµΩ∆Ω∫‚∫Û£¨»ÙCO2◊™ªØ¬ Œ™a£¨‘Ú»ð∆˜ƒ⁄µƒ—π«ø”Î∆ º—π«ø÷Ʊ»Œ™______________°£
¢Ú.Ω´∑œÀÆ÷–µƒ”–∫¶ŒÔ÷ ◊™±‰Œ™ŒÞ∂æŒÔ÷ °¢ƒ—»ÐŒÔ÷ º∞“◊≥˝»•µƒŒÔ÷ £¨ «∑œÀÆ¥¶¿Ì÷–µƒ÷ÿ“™∑Ω∑®°£
£®5£©∫¨«Ë∑œÀÆ÷–µƒ«ËªØŒÔ≥£“‘[Fe(CN)6]3-∫ÕCN-µƒ–Œ Ω¥Ê‘⁄£¨π§“µ…œ”–∂ý÷÷∑œÀÆ¥¶¿Ì∑Ω∑®°£∆‰÷–µÁΩ‚¥¶¿Ì∑®»ÁÕº£∫”√»ÁÕºÀ˘ æ◊∞÷√¥¶¿Ì∫¨CN-∑œÀÆ ±£¨øÿ÷∆»Ð“∫pHŒ™9~10≤¢º”»Î“ª∂®¡øµƒNaCl£¨“ª∂®Ãıº˛œ¬µÁΩ‚£¨—Ùº´≤˙…˙ClO-µƒΩ´CN-—ıªØŒ™ŒÞ∫¶ŒÔ÷ ∂¯≥˝»•°£Ã˙µÁº´Œ™_____________£®ÃÓ°∞“ıº´°±ªÚ°∞—Ùº´°±£©£¨—Ùº´≤˙…˙µƒClO-µƒµÁº´∑¥”¶Œ™_______________________£¨—Ùº´≤˙…˙µƒClO-µƒΩ´CN-—ıªØŒ™ŒÞ∫¶ŒÔ÷ ∂¯≥˝»•µƒ¿Î◊”∑Ω≥Ã ΩŒ™___________________________°£

°æ¥∞∏°ø 2858 CH3OH(l)+O2(g) =CO(g)+2H2O (g) °˜H=-443.5kJmol-1 ¢€¢Ð 1-0.5a “ıº´ Cl-+2OH--2e-=ClO-+H2O 2CN-+5C1O-+2OH-=N2°¸+5Cl-+2CO32-+H2O
°æΩ‚Œˆ°ø¢Ò. ‘Â∑÷Œˆ£∫ø∆—ߺ“¿˚”√ô—ÙƒÐ∑÷Ω‚ÀÆ…˙≥…µƒ«‚∆¯‘⁄¥þªØº¡◊˜”√œ¬”Î∂˛—ıªØú∑¥”¶…˙≥…º◊¥º£¨≤¢ø™∑¢≥ˆ÷±Ω”“‘º◊¥ºŒ™»º¡œµƒ»º¡œµÁ≥ÿ°£º∫÷™H2(g)°¢CO(g)∫ÕCH3OH(l)µƒ»º…’»»¶§H∑÷±Œ™-285.8kJ°§mol-1°¢-283.0 kJ°§mol-1 ∫Õ-726.5 kJ°§mol-1°£‘ÚH2»º…’µƒ»»ªØ—ß∑Ω≥Ã ΩŒ™H2(g) + ![]() O2(g)= H2O(l) ¶§H=-285.8kJ°§mol-1£ªCO(g)»º…’µƒ»»ªØ—ß∑Ω≥Ã ΩŒ™CO(g)+
O2(g)= H2O(l) ¶§H=-285.8kJ°§mol-1£ªCO(g)»º…’µƒ»»ªØ—ß∑Ω≥Ã ΩŒ™CO(g)+ ![]() O2(g)= CO2 (g) ¶§H=-283.0kJ°§mol-1£ªCH3OH(l) +
O2(g)= CO2 (g) ¶§H=-283.0kJ°§mol-1£ªCH3OH(l) +![]() O2(g)=2 H2O(l)+ CO2 (g) ¶§H=-726.5kJ°§mol-1.
O2(g)=2 H2O(l)+ CO2 (g) ¶§H=-726.5kJ°§mol-1.
£®1£©”…«‚∆¯µƒ»º…’»»ø…÷™£¨H2O(l) =H2(g)+ ![]() O2(g) ¶§H=+285.8kJ°§mol-1£¨À˘“‘”√ô—ÙƒÐ∑÷Ω‚10molÀÆœ˚∫ƒµƒƒÐ¡ø «2858kJ°£
O2(g) ¶§H=+285.8kJ°§mol-1£¨À˘“‘”√ô—ÙƒÐ∑÷Ω‚10molÀÆœ˚∫ƒµƒƒÐ¡ø «2858kJ°£
£®2£©”…CO(g)+ ![]() O2(g)= CO2 (g) ¶§H=-283.0kJ°§mol-1°¢CH3OH(l) +
O2(g)= CO2 (g) ¶§H=-283.0kJ°§mol-1°¢CH3OH(l) +![]() O2(g)=2H2O(l)+ CO2 (g) ¶§H=-726.5kJ°§mol-1≤¢∏˘æð∏«Àπ∂®¬…ø…÷™£¨º◊¥º≤ªÕÍ»´»º…’…˙≥…“ª—ıªØú∫Õ“∫èÀƵƒ»»ªØ—ß∑Ω≥Ã ΩŒ™CH3OH(l)+O2(g) =CO(g)+2H2O (g) °˜H=-443.5kJmol-1°£
O2(g)=2H2O(l)+ CO2 (g) ¶§H=-726.5kJ°§mol-1≤¢∏˘æð∏«Àπ∂®¬…ø…÷™£¨º◊¥º≤ªÕÍ»´»º…’…˙≥…“ª—ıªØú∫Õ“∫èÀƵƒ»»ªØ—ß∑Ω≥Ã ΩŒ™CH3OH(l)+O2(g) =CO(g)+2H2O (g) °˜H=-443.5kJmol-1°£
£®3£©‘⁄»ðª˝Œ™2Lµƒ√б’»ð∆˜÷–£¨”…CO2∫ÕH2∫œ≥…º◊¥º£¨±£≥÷∆‰À˚Ãıº˛≤ª±‰£¨ÃΩæøŒ¬∂»∂‘∑¥”¶µƒ”∞œÏ°£”…Õºø…÷™£¨T2 ±∑¥”¶µΩ¥Ô∆Ω∫‚ΩœøÏ£¨π T2£æT1£ªŒ¬∂»…˝∏þ∫Ûº◊¥ºµƒŒÔ÷ µƒ¡øºı…Ÿ£¨‘Ú∏√∑¥”¶Œ™∑≈»»∑¥”¶£¨Œ¬∂»‘Ω∏þ∆‰∆Ω∫‚≥£ ˝‘Ω–°°£¢ŸŒ¬∂»Œ™T1 ±£¨¥”∑¥”¶ø™ ºµΩ∆Ω∫‚£¨![]() ƒ⁄º◊¥ºµƒ±‰ªØ¡øŒ™
ƒ⁄º◊¥ºµƒ±‰ªØ¡øŒ™![]() £¨‘Ú…˙≥…º◊¥ºµƒ∆Ωæ˘ÀŸ¬ Œ™v(CH3OH) =
£¨‘Ú…˙≥…º◊¥ºµƒ∆Ωæ˘ÀŸ¬ Œ™v(CH3OH) =![]() mol°§L-1°§min-1£¨¢Ÿ≤ª’˝»∑£ª¢⁄∏√∑¥”¶‘⁄T1 ±µƒ∆Ω∫‚≥£ ˝±»T2 ±µƒ¥Û£¨¢⁄≤ª’˝»∑£ª¢€∏√∑¥”¶Œ™∑≈»»∑¥”¶£¨¢€’˝»∑£ª¢ÐAµ„Œ™T1Œ¬∂»œ¬µƒ∆Ω∫‚◊¥Ã¨£¨¥¶”⁄Aµ„µƒ∑¥”¶ÃÂœµ¥”T1±‰µΩT2£¨∆Ω∫‚œÚƒÊ∑¥”¶∑ΩœÚ“∆∂Ø£¨π ¥ÔµΩ∆Ω∫‚ ±
mol°§L-1°§min-1£¨¢Ÿ≤ª’˝»∑£ª¢⁄∏√∑¥”¶‘⁄T1 ±µƒ∆Ω∫‚≥£ ˝±»T2 ±µƒ¥Û£¨¢⁄≤ª’˝»∑£ª¢€∏√∑¥”¶Œ™∑≈»»∑¥”¶£¨¢€’˝»∑£ª¢ÐAµ„Œ™T1Œ¬∂»œ¬µƒ∆Ω∫‚◊¥Ã¨£¨¥¶”⁄Aµ„µƒ∑¥”¶ÃÂœµ¥”T1±‰µΩT2£¨∆Ω∫‚œÚƒÊ∑¥”¶∑ΩœÚ“∆∂Ø£¨π ¥ÔµΩ∆Ω∫‚ ±![]() ‘ˆ¥Û£¨¢Ð’˝»∑°£◊€…œÀ˘ ˆ£¨Àµ∑®’˝»∑µƒ «¢€¢Ð°£
‘ˆ¥Û£¨¢Ð’˝»∑°£◊€…œÀ˘ ˆ£¨Àµ∑®’˝»∑µƒ «¢€¢Ð°£
£®4£©‘⁄T1Œ¬∂» ±£¨Ω´1mol CO2∫Õ3mol H2≥‰»Î“ª√б’∫„»ð»ð∆˜÷–£¨∑¢…˙µƒ∑¥”¶Œ™CO2 (g)£´3H2 (g) CH3OH (g)£´H2O (g)£¨≥‰∑÷∑¥”¶¥ÔµΩ∆Ω∫‚∫Û£¨»ÙCO2◊™ªØ¬ Œ™a£¨‘ÚCO2°¢H2°¢CH3OH °¢H2Oµƒ±‰ªØ¡ø∑÷±Œ™a mol°¢3amol°¢a mol°¢a mol£¨∆Ω∫‚ ±∆¯Ãµƒ◊ÐŒÔ÷ µƒ¡øŒ™(4-2a) mol£®◊¢“‚∏þ”⁄300°Ê ±£¨CH3OH «∆¯Ã£©£¨‘Ú»ð∆˜ƒ⁄µƒ—π«ø”Î∆ º—π«ø÷Ʊ»µ»”⁄∆‰ŒÔ÷ µƒ¡ø÷Ʊ»£¨Œ™![]() 1-0.5a°£
1-0.5a°£
¢Ú.£®5£©”…“‚ø…÷™£¨”√»ÁÕºÀ˘ æ◊∞÷√¥¶¿Ì∫¨CN-∑œÀÆ ±£¨øÿ÷∆»Ð“∫pHŒ™9~10º”»Î“ª∂®¡øµƒNaCl£¨“ª∂®Ãıº˛œ¬µÁΩ‚£¨—Ùº´…œ¬»¿Î◊”∑≈µÁ£¨¬»¿Î◊”±ª—ıªØŒ™ClO-£¨≤˙…˙ClO-µƒΩ´CN-—ıªØŒ™N2∂¯≥˝»•£¨À˘“‘Ã˙µÁº´≤ªø…ƒÐ «—Ùº´£¨Ã˙µÁº´Œ™“ıº´£¨—Ùº´≤˙…˙µƒClO-µƒµÁº´∑¥”¶Œ™Cl-+2OH--2e-=ClO-+H2O£¨—Ùº´≤˙…˙µƒClO-µƒΩ´CN-—ıªØŒ™N2µƒ¿Î◊”∑Ω≥Ã ΩŒ™2CN-+5C1O-+2OH-=N2°¸+5Cl-+2CO32-+H2O°£

 ÃÏÃϜڅœ“ª±æ∫√æÌœµ¡–¥∞∏
ÃÏÃϜڅœ“ª±æ∫√æÌœµ¡–¥∞∏ –°—ß…˙10∑÷÷””¶”√Âœµ¡–¥∞∏
–°—ß…˙10∑÷÷””¶”√Âœµ¡–¥∞∏°æƒø°ø¥øºÓ «÷ÿ“™µƒªØπ§‘≠¡œ£¨‘⁄“Ω“©°¢“±Ω°¢ªØπ§°¢ ≥∆∑µ»¡Ï”Ú±ªπ„∑∫ π”√°£
I. ”√¥ø檵ƒÃºÀ·ƒ∆πÃÃÂ≈‰÷∆500mL 0.40mol/L Na2CO3»Ð“∫°£
(1)≥∆»°Na2CO3πÃõƒ÷ ¡ø «______________________g°£
(2)≈‰÷∆»Ð“∫ ±£¨Ω¯––»Áœ¬≤Ÿ◊˜£¨∞¥’’≤Ÿ◊˜À≥–Ú£¨µ⁄4≤Ω «_________(ÃÓ◊÷ƒ∏)°£
a. ∂®»ð b. º∆À„ c. »ÐΩ‚ d. “°‘» e. ◊™“∆ f. œ¥µ” g. ≥∆¡ø
(3)œ¬¡–Àµ∑®÷–£¨’˝»∑µƒ «_____________________(ÃÓ◊÷ƒ∏)°£
a. ∂®»ð ±£¨—ˆ ”øÃ∂»œþ£¨ª·µº÷¬≈‰÷∆µƒ»Ð“∫≈®∂»∆´–°
b. ∂®»ð ±£¨»Áπ˚º”ÀÆ≥¨π˝øÃ∂»œþ£¨“™”√µŒπÐŒ¸≥ˆ
c. ◊™“∆ ±£¨»Ð“∫µπ≥ˆ»ð¡ø∆øÕ‚£¨“™÷ÿ–¬≈‰÷∆»Ð“∫
d. “°‘»∫Û£¨“∫√ʵՔ⁄øÃ∂»œþ£¨“™‘Ÿº”ÀÆ÷¡øÃ∂»œþ
II. ƒ≥ µ—È–°◊ȵƒÕ¨—߃£ƒ‚∫Óµ¬∞Ò÷∆ºÓ∑®÷∆»°¥øºÓ£¨¡˜≥ûÁœ¬£∫

(1)𧓵…˙≤˙¥øºÓµƒµ⁄“ª≤Ω «≥˝»•±•∫Õ ≥—ŒÀƵƒ÷–SO42®D°¢Ca2+¿Î◊”£¨“¿¥Œº”»Îµƒ ‘º¡º∞∆‰”√¡ø « ______________°¢ _______________°¢ (π˝¬À)°¢ _______________°£
(2)“—÷™£∫º∏÷÷—Œµƒ»ÐΩ‚∂»
NaCl | NH4HCO3 | NaHCO3 | NH4Cl | |
»ÐΩ‚∂»(20°„C£¨100gH2O ±) | 36.0 | 21.7 | 9.6 | 37.2 |
¢Ÿ–¥≥ˆ◊∞÷√I÷–∑¥”¶µƒªØ—ß∑Ω≥à Ω________________________________________°£
¢⁄–¥≥ˆ◊∞÷√II÷–∑¢…˙∑¥”¶µƒªØ—ß∑Ω≥à Ω________________________________°£
(3)∏√¡˜≥Ã÷–ø…—≠ª∑¿˚”√µƒŒÔ÷ «__________________°£
(4)÷∆≥ˆµƒ¥øºÓ÷–÷ª∫¨”–‘”÷ NaCl°£
¢ŸºÏ—È”√∏√¥øºÓ≈‰÷∆µƒ»Ð“∫÷–∫¨”–Cl®Dµƒ∑Ω∑® «_________________________°£
¢⁄≤‚∂®∏√¥øºÓµƒ¥ø∂»£¨œ¬¡–∑Ω∞∏÷–ø…––µƒ «__________(ÃÓ◊÷ƒ∏)°£
a. œÚmøÀ¥øºÓ—˘∆∑÷–º”»Î◊„¡øCaCl2»Ð“∫£¨≥¡µÌæ≠π˝¬À°¢œ¥µ”°¢∏…‘Ô£¨≥∆∆‰÷ ¡øŒ™b g
b. œÚmøÀ¥øºÓ—˘∆∑÷–º”»Î◊„¡øœ°—ŒÀ·£¨”√ºÓ ت“(÷˜“™≥…∑÷ «CaO∫ÕNaOH)Œ¸ ’≤˙…˙µƒ∆¯Ã£¨ºÓ ت“‘ˆ÷ÿb g
c. œÚmøÀ¥øºÓ—˘∆∑÷–º”»Î◊„¡øAgNO3»Ð“∫£¨≤˙…˙µƒ≥¡µÌæ≠π˝¬À°¢œ¥µ”°¢∏…‘Ô£¨≥∆∆‰÷ ¡øŒ™b g
°æƒø°øCO2µƒ¿˚”√ «π˙º …Áª·∆’±Èπÿ◊¢µƒŒ °£
£®1£©CO2µƒµÁ◊” Ω «______°£
£®2£©CO2‘⁄¥þªØº¡◊˜”√œ¬ø…“‘÷±Ω”◊™ªØŒ™““∂˛¥º∫Õº◊¥º£¨µ´»Ù∑¥”¶Œ¬∂»π˝∏þ£¨““∂˛¥ºª·…Ó∂»º”«‚…˙≥…““¥º°£
![]()
ªÒ»°““∂˛¥ºµƒ∑¥”¶¿˙≥Ãø…∑÷Œ™»Áœ¬2≤Ω£∫
¢Ò£Æ
¢Ú£ÆECº”«‚…˙≥…““∂˛¥º”κ◊¥º
![]()
¢Ÿ ≤Ω÷Ë¢Úµƒ»»ªØ—ß∑Ω≥Ã Ω «______°£
¢⁄ —–æø∑¥”¶Œ¬∂»∂‘ECº”«‚µƒ”∞œÏ£®∑¥”¶ ±º‰æ˘Œ™4–° ±£©£¨ µ—È ˝æðº˚œ¬±Ì£∫
∑¥”¶Œ¬∂»/ °Ê | EC◊™ªØ¬ / % | ≤˙¬ / % | |
““∂˛¥º | º◊¥º | ||
160 | 23.8 | 23.2 | 12.9 |
180 | 62.1 | 60.9 | 31.5 |
200 | 99.9 | 94.7 | 62.3 |
220 | 99.9 | 92.4 | 46.1 |
”……œ±Ìø…÷™£¨Œ¬∂»‘Ω∏þ£¨ECµƒ◊™ªØ¬ ‘Ω∏þ£¨‘≠“Ú «______°£Œ¬∂»…˝∏þµΩ220 °Ê ±£¨““∂˛¥ºµƒ≤˙¬ ∑¥∂¯ΩµµÕ£¨‘≠“Ú «______°£
£®3£©”√œ°¡ÚÀ·◊˜µÁΩ‚÷ »Ð“∫£¨µÁΩ‚CO2ø…÷∆»°º◊¥º£¨◊∞÷√»Áœ¬ÕºÀ˘ 棨µÁº´aΩ”µÁ‘¥µƒ______º´£®ÃÓ°∞’˝°±ªÚ°∞∏∫°±£©£¨…˙≥…º◊¥ºµƒµÁº´∑¥”¶ Ω «______°£

£®4£©CO2ΩœŒ»∂®°¢ƒÐ¡øµÕ°£Œ™ µœ÷CO2µƒªØ—ß¿˚”√£¨œ¬¡–—–æø∑ΩœÚ∫œ¿Ìµƒ «______£®ÃÓ–Ú∫≈£©°£
a£Æ—°‘Ò∏þƒÐ¡øµƒ∑¥”¶ŒÔ∫ÕCO2∑¥”¶ªÒµ√µÕƒÐ¡øµƒ…˙≥…ŒÔ
b£Æ¿˚”√µÁƒÐ°¢π‚ƒÐªÚ»»ƒÐªÓªØCO2∑÷◊”
c£Æ—°‘Ò∏þ–ßµƒ¥þªØº¡
