��Ŀ����
A��B��C��D��E������Һ�ֱ���HCl��CH3COOH��NaOH��NH3?H2O��Na2CO3��Һ�е�һ�֣������½�������ʵ�飺
�ٽ�1L pH=9��A��Һ�ֱ���xL 0.001mol/L B��Һ��y L 0.001m/L D��Һ��ַ�Ӧ����Һ�����ԣ�x��y��С��ϵΪ��y��x��
��Ũ�Ⱦ�Ϊ0.1mol/L C��D��Һ�������ϣ���Һ�����ԣ�
��Ũ�Ⱦ�Ϊ0.1mol/L A��E��Һ��pH��A��E��
�ش��������⣺
��1��D�� ��Һ���ѧʽ����
��2��д��A������B��Һ��Ӧ�����ӷ���ʽ ��
��3����������������ʵ���Ũ�ȵ�D��E�ֱ������������۷�Ӧ������H2�����ʵ���֮��Ϊ ��
��4����������������ʵ���Ũ�ȵ�B��C��Ϻ�������Һ��pHԼΪ7��ԭ���� ��
��5������ʱ��һ�����0.4mol/L��E��Һ�У�����һ�������0.1mol/L��D��Һʱ�������ҺpH=13������Ϻ���Һ������仯���Բ��ƣ���D��E��Һ�����֮���� ��
�ٽ�1L pH=9��A��Һ�ֱ���xL 0.001mol/L B��Һ��y L 0.001m/L D��Һ��ַ�Ӧ����Һ�����ԣ�x��y��С��ϵΪ��y��x��
��Ũ�Ⱦ�Ϊ0.1mol/L C��D��Һ�������ϣ���Һ�����ԣ�
��Ũ�Ⱦ�Ϊ0.1mol/L A��E��Һ��pH��A��E��
�ش��������⣺
��1��D��
��2��д��A������B��Һ��Ӧ�����ӷ���ʽ
��3����������������ʵ���Ũ�ȵ�D��E�ֱ������������۷�Ӧ������H2�����ʵ���֮��Ϊ
��4����������������ʵ���Ũ�ȵ�B��C��Ϻ�������Һ��pHԼΪ7��ԭ����
��5������ʱ��һ�����0.4mol/L��E��Һ�У�����һ�������0.1mol/L��D��Һʱ�������ҺpH=13������Ϻ���Һ������仯���Բ��ƣ���D��E��Һ�����֮����
������A��pH����7��˵���ʼ��ԣ����ݢ�ȷ��B��DΪHCl��CH3COOH�����ᣬ����ͬŨ��ʱB��������˵��B�����ἴΪCH3COOH����DΪHCl��
C��D��Һ�������Ϻ���Һ�����ԣ������ɵ�����ǿ�������Σ����ݢ�ȷ��C�����ΪNH3?H2O��Ũ�Ⱦ�Ϊ0.1 mol/L��C��D��Һ�������Ϻ���Һ�����ԣ����Ȼ��ˮ������ԣ�
��ͬŨ��ʱ��ǿ��ļ��Դ����εļ��ԣ����ݢ�ȷ��A��E�ֱ���NaOH��Na2CO3��0.1 mol/L��A��ҺpHС��0.1 mol/L��E��ҺpH˵��E��ǿ��NaOH��A��Na2CO3��
C��D��Һ�������Ϻ���Һ�����ԣ������ɵ�����ǿ�������Σ����ݢ�ȷ��C�����ΪNH3?H2O��Ũ�Ⱦ�Ϊ0.1 mol/L��C��D��Һ�������Ϻ���Һ�����ԣ����Ȼ��ˮ������ԣ�
��ͬŨ��ʱ��ǿ��ļ��Դ����εļ��ԣ����ݢ�ȷ��A��E�ֱ���NaOH��Na2CO3��0.1 mol/L��A��ҺpHС��0.1 mol/L��E��ҺpH˵��E��ǿ��NaOH��A��Na2CO3��
����⣺A��pH����7��˵���ʼ��ԣ����ݢ�ȷ��B��DΪHCl��CH3COOH�����ᣬ����ͬŨ��ʱB��������˵��B�����ἴΪCH3COOH����DΪHCl��
C��D��Һ�������Ϻ���Һ�����ԣ������ɵ�����ǿ�������Σ����ݢ�ȷ��C�����ΪNH3?H2O��Ũ�Ⱦ�Ϊ0.1 mol/L��C��D��Һ�������Ϻ���Һ�����ԣ����Ȼ��ˮ������ԣ�
��ͬŨ��ʱ��ǿ��ļ��Դ����εļ��ԣ����ݢ�ȷ��A��E�ֱ���NaOH��Na2CO3��0.1 mol/L��A��ҺpHС��0.1 mol/L��E��ҺpH˵��E��ǿ��NaOH��A��Na2CO3��
��1��ͨ�����Ϸ���֪��D��HCl��Һ��
�ʴ�Ϊ��HCl��
��2�������̼���Ʒ�Ӧ���ӷ���ʽΪ��2CH3COOH+CO32-�T2CH3COO-+CO2��+H2O��
�ʴ�Ϊ��2CH3COOH+CO32-�T2CH3COO-+CO2��+H2O��
��3��DΪ���ᣬE���������ƣ������ʵ�����D��E�ֱ������������۷�Ӧ��2Al+2NaOH+2H2O�T2NaAlO2+3H2����2Al+6HCl�T2AlCl3+3H2�������ݷ���ʽ֪�������ʵ�����������������Ʒֱ�����������Ӧ����H2�����ʵ���֮��Ϊ1��3��
�ʴ�Ϊ��1��3��
��4��BΪ���ᣬC�ǰ�ˮ����������������ʵ���Ũ�ȵ�B��C��Ϻ����ǡ�÷�Ӧ���ɴ���泥�������Һ��pHԼΪ7��ԭ���Ǵ�������ӵ�ˮ��̶���笠����ӵ�ˮ��̶��൱��
�ʴ�Ϊ����������ӵ�ˮ��̶���笠����ӵ�ˮ��̶��൱��
��5������ʱ��һ�����0.4mol/L������������Һ�У�����һ�������0.1mol/L��������Һʱ�������ҺpH=13��pH=13������Һ������������Ũ��Ϊ0.1mol/L��
���������Ƶ������x������������y��
�����Һ������������Ũ��=
mol/L=0.1mol/L��
y��x=3��2��
����������������Ƶ����֮��=3��2��
�ʴ�Ϊ��3��2��
C��D��Һ�������Ϻ���Һ�����ԣ������ɵ�����ǿ�������Σ����ݢ�ȷ��C�����ΪNH3?H2O��Ũ�Ⱦ�Ϊ0.1 mol/L��C��D��Һ�������Ϻ���Һ�����ԣ����Ȼ��ˮ������ԣ�
��ͬŨ��ʱ��ǿ��ļ��Դ����εļ��ԣ����ݢ�ȷ��A��E�ֱ���NaOH��Na2CO3��0.1 mol/L��A��ҺpHС��0.1 mol/L��E��ҺpH˵��E��ǿ��NaOH��A��Na2CO3��
��1��ͨ�����Ϸ���֪��D��HCl��Һ��
�ʴ�Ϊ��HCl��
��2�������̼���Ʒ�Ӧ���ӷ���ʽΪ��2CH3COOH+CO32-�T2CH3COO-+CO2��+H2O��
�ʴ�Ϊ��2CH3COOH+CO32-�T2CH3COO-+CO2��+H2O��
��3��DΪ���ᣬE���������ƣ������ʵ�����D��E�ֱ������������۷�Ӧ��2Al+2NaOH+2H2O�T2NaAlO2+3H2����2Al+6HCl�T2AlCl3+3H2�������ݷ���ʽ֪�������ʵ�����������������Ʒֱ�����������Ӧ����H2�����ʵ���֮��Ϊ1��3��
�ʴ�Ϊ��1��3��
��4��BΪ���ᣬC�ǰ�ˮ����������������ʵ���Ũ�ȵ�B��C��Ϻ����ǡ�÷�Ӧ���ɴ���泥�������Һ��pHԼΪ7��ԭ���Ǵ�������ӵ�ˮ��̶���笠����ӵ�ˮ��̶��൱��
�ʴ�Ϊ����������ӵ�ˮ��̶���笠����ӵ�ˮ��̶��൱��
��5������ʱ��һ�����0.4mol/L������������Һ�У�����һ�������0.1mol/L��������Һʱ�������ҺpH=13��pH=13������Һ������������Ũ��Ϊ0.1mol/L��
���������Ƶ������x������������y��
�����Һ������������Ũ��=
| 0.4��x-0.1y |
| x+y |
y��x=3��2��
����������������Ƶ����֮��=3��2��
�ʴ�Ϊ��3��2��
���������⿼���������ʵĵ��룬��ȷ�ƶ������ǽⱾ��ؼ���������ʵ���������������Ѷ��еȣ�

��ϰ��ϵ�д�
 ����ѧУ�ֲ����ܲ�ϵ�д�
����ѧУ�ֲ����ܲ�ϵ�д� �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�����Ŀ



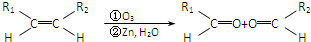

 Ԫ�����ڱ������ڵ�һ���������ʾ�������й�A��B��C��D��E����Ԫ�ص������У���ȷ���ǣ�������
Ԫ�����ڱ������ڵ�һ���������ʾ�������й�A��B��C��D��E����Ԫ�ص������У���ȷ���ǣ�������