��Ŀ����
����Ŀ����(N2H4)�ڲ�ͬ�����·ֽ���ﲻͬ��200 ��ʱ��Cu����ֽ�Ļ�����ͼ����ʾ����֪200 ��ʱ��
��Ӧ��3N2H4(g)===N2(g)��4NH3(g) ��H1����32.9 kJ��mol��1
��Ӧ��N2H4(g)��H2(g)===2NH3(g) ��H2����41.8 kJ��mol��1
����˵���в���ȷ���� (�� ��)
A.ͼ��ʾ���̢١��ڶ��Ƿ��ȷ�Ӧ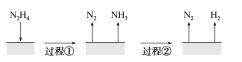
B.��Ӧ�����������ʾ��ͼ��ͼ��ʾ
C.����3 mol N2H4(g)�еĻ�ѧ�����յ�����С���γ�1 mol N2(g)��4 mol NH3(g)�еĻ�ѧ���ͷŵ�����
D.200 ��ʱ���·ֽ����ɵ������������Ȼ�ѧ����ʽΪN2H4(g)===N2(g)��2H2(g)����H����50.7 kJ��mol��1
���𰸡�A
��������
���������֪�����⿼�鷴Ӧ�Ⱥ��ʱ�ļ��㣬���ø�˹���ɷ�����
A.���̢���N2H4�ֽ�����N2��NH3,��֪�Ȼ�ѧ����ʽI�С�HΪ��ֵ,����ͼʾ���̢�Ϊ���ȷ�Ӧ,���̢ڸ��ݸ�˹���ɣ�(I)2��(II)��N2H4(g)�TN2(g)+2H2(g)��H�T32.9kJmol12��(41.8kJmol1)=+50.7kJmol1��Ϊ���ȷ�Ӧ��A�����
B.��ӦII:N2H4(g)+H2(g)�T2NH3(g)��H2=41.8KJ/mol����ӦΪ���ȷ�Ӧ����Ӧ���������������B����ȷ��
C.��ӦI:3N2H4(g)�TN2(g)+4NH3(g)��Hl=32.9KJ/mol,��ӦΪ���ȷ�Ӧ,�Ͽ�3molN2H4(g)�еĻ�ѧ�����յ�����С���γ�1moIN2(g)��4molNH3(g)�еĻ�ѧ���ͷŵ�������C����ȷ��
D.���ݸ�˹���ɣ�(I)2��(II)��N2H4(g)�TN2(g)+2H2(g)��H�T32.9kJmol12��(41.8kJmol1)=+50.7kJmol1��D����ȷ��
��ѡA��

 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д�����Ŀ��I.�������������������������ܹ������練Ӧ��2MnO4��+5H2C2O4+6H��=2Mn2��+10CO2��+8H2O���� 4mL 0.001mol/L KMnO4 ��Һ��2mL 0.01mol/L H2C2O4 ��Һ���о���ͬ�����Ի�ѧ��Ӧ���ʵ�Ӱ�죮�ı���������£�
��� | 10%�������/mL | �¶�/�� | �������� |
�� | 2mL | 20 | |
�� | 2mL | 20 | 10 �α��� MnSO4 ��Һ |
�� | 2mL | 30 | |
�� | 1mL | 20 | 1mL ����ˮ |
��1���÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ_____
��2������о������Ի�ѧ��Ӧ���ʵ�Ӱ�죬ʹ��ʵ��_________��_____������������ʾ����ͬ���� ����о��¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�죬ʹ��ʵ��_____��_________��
��3���Ա�ʵ���������������о�_____�Ի�ѧ��Ӧ���ʵ�Ӱ�죬ʵ�����м��� 1mL����ˮ��Ŀ����_______
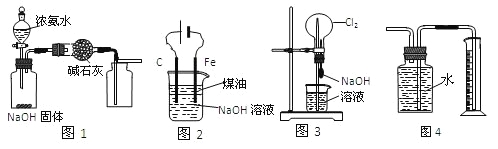
II��������ͼװ�ò����ʵ��Լ������ij̽��ʵ�飬���ó���Ӧʵ����ۣ�����������Ϣ�ش�

��1��Ϊ��֤��Ԫ�صķǽ�����ǿ���� S��C��Si������Ϊ������Ӧ���ǣ� ��Ϊ______����Ϊ______����Ϊ______������֪���������ݲ��������� �а�ɫ������
��2�������Ϊˮ����Ϊ Na2O2 ��ĩ����Ϊ H2S �ı���ˮ��Һ��ʵ���й۲쵽�������ɵ���ɫ������˵��Ԫ�أϡ��ӵõ�������ǿ��Ϊ ______��
��3������װ�����Ӻú��ڼ���ҩƷ��ʼʵ��ǰ������������Լ�飬��������ò���_____��
����Ŀ�����Ļ����������������й㷺���ڡ�
(1)���Ȱ���NH2Cl���ĵ���ʽΪ________����ͨ����ӦNH3(g)��Cl2(g)=NH2Cl(g)��HCl(g)�Ʊ��Ȱ�����֪���ֻ�ѧ���ļ������ұ���ʾ���ٶ���ͬ������ͬ�ֻ�ѧ���ļ���һ��������������Ӧ�Ħ�H=_________��
��ѧ�� | ����/(kJ��mol-1) |
N-H | 391.3 |
Cl-Cl | 243.0 |
N-Cl | 191.2 |
H-Cl | 431.8 |
��NH2Cl��ˮ��Ӧ����ǿ�����Ե����ʣ�������Ч�������������÷�Ӧ�Ļ�ѧ����ʽΪ________��
(2)�ý�̿��ԭNO�ķ�ӦΪ��2NO(g)+C(s)![]() N2(g)+CO2(g)�����ݻ���Ϊ1L�ļס��ҡ����������ݺ��£���Ӧ�¶ȷֱ�Ϊ400�桢400�桢T�棩�����зֱ���������Ľ�̿��һ������NO����ø�������n(NO)�淴Ӧʱ��t�ı仯������±���ʾ��
N2(g)+CO2(g)�����ݻ���Ϊ1L�ļס��ҡ����������ݺ��£���Ӧ�¶ȷֱ�Ϊ400�桢400�桢T�棩�����зֱ���������Ľ�̿��һ������NO����ø�������n(NO)�淴Ӧʱ��t�ı仯������±���ʾ��
t/min | 0 | 40 | 80 | 120 | 160 |
n(NO)����������/mol | 2.00 | 1.50 | 1.10 | 0.80 | 0.80 |
n(NO)����������/mol | 1.00 | 0.80 | 0.65 | 0.53 | 0.45 |
n(NO)����������/mol | 2.00 | 1.45 | 1.00 | 1.00 | 1.00 |
�ٸ÷�ӦΪ____________������ȡ������ȡ�����Ӧ��
����������200min�ﵽƽ��״̬����0��200min����NO��Ũ�ȱ仯��ʾ��ƽ����Ӧ����v(NO)=_________��
(3)�ý�̿��ԭNO2�ķ�ӦΪ��2NO2(g)+2C(s) ![]() N2(g)+2CO2(g)���ں��������£�1molNO2������C�����÷�Ӧ�����ƽ��ʱNO2��CO2�����ʵ���Ũ����ƽ����ѹ�Ĺ�ϵ��ͼ��ʾ��
N2(g)+2CO2(g)���ں��������£�1molNO2������C�����÷�Ӧ�����ƽ��ʱNO2��CO2�����ʵ���Ũ����ƽ����ѹ�Ĺ�ϵ��ͼ��ʾ��

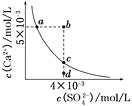
��A��B�����Ũ��ƽ�ⳣ����ϵ��Kc(A)_____Kc(B)�������������=������
��A��B��C������NO2��ת������ߵ���______���A����B����C�����㡣
�ۼ���C��ʱ�÷�Ӧ��ѹǿƽ�ⳣ��Kp(C)=______��Kp����ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ����ѹ�����ʵ�����������