��Ŀ����
ij��ɫ��Һ�������п��ܴ��ڵ��������£�Na+��Ag+��Ba2+��Al3+��AlO2-��CO32-��SiO32-��SO32-��SO42-����ȡ����Һ�����й�ʵ�飬ʵ������ͼ��ʾ������˵����ȷ���ǣ�������
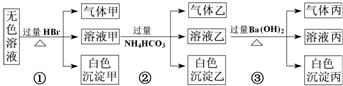

| A����ʵ��ٿ�֪�������һ���Ǵ����� | B����ʵ��ڿ�֪��������һ����CO2����������Al��OH��3 ԭ��Һ�п϶�����Al3+ | C����ʵ��ۿ�֪���������NH3��������һ����BaCO3��������BaSO4 | D��ԭ��Һ�п϶�����SiO32-��AlO2-��Na+��CO32-������ȷ���Ƿ���SO32-��SO42- |
�������ټ���������а�ɫ�������ɣ��ð�ɫ������ֻ��Ϊ���ᣬ������Һ�к���SiO32-�����ܴ���Ag+��Ba2+��Al3+��������Һ�����Կ�֪���϶���Na+�������ΪCO2��SO2������һ�֣�CO32-��SO32-������һ�֣�
�ڼ�̼����狀���г����������壬˵����Һ����Al3+����ԭ��Һ��AlO2-��HBr��Ӧ��ת��ΪAl3+������ΪAl��OH��3������ΪCO2��
����ʵ��ۣ�������̼�������Ba��OH��2��Ӧ����������NH3������һ����BaCO3��������ȷ����û��BaSO4��
�������Ϸ������н��
�ڼ�̼����狀���г����������壬˵����Һ����Al3+����ԭ��Һ��AlO2-��HBr��Ӧ��ת��ΪAl3+������ΪAl��OH��3������ΪCO2��
����ʵ��ۣ�������̼�������Ba��OH��2��Ӧ����������NH3������һ����BaCO3��������ȷ����û��BaSO4��
�������Ϸ������н��
����⣺����ʵ��ٿ�֪���ð�ɫ������ֻ��Ϊ���ᣬ������Һ�к���SiO32-�����ܴ������������ӷ�Ӧ�����ӣ�Ag+��Ba2+��Al3+���ٸ��ݸ�����Һ�����Կ�֪��ԭ��Һ�п϶�����Ψһ��������Na+�������ΪCO2��SO2������һ�֣���CO32-��SO32-������һ�֣�
����ʵ��ڼ�̼����狀���г����������壬˵����Һ����Al3+����ԭ��Һ��AlO2-��HBr��Ӧ��ת��ΪAl3+������ΪAl��OH��3������ΪCO2��
����ʵ��ۣ�������̼�������Ba��OH��2��Ӧ����������NH3������һ����BaCO3��������ȷ����û��BaSO4��
A���������Ϸ�����֪���������Ϊ������̼�Ͷ�������Ļ�����A����
B��ʵ��ڼ�̼����狀���г����������壬˵����Һ����Al3+����ԭ��Һ��AlO2-��HBr��Ӧ��ת��ΪAl3+������ΪAl��OH��3������ΪCO2��ԭ��Һ�д��ڵ���AlO2-����B����
C����ʵ��ۣ�������̼�������Ba��OH��2��Ӧ����������NH3��������һ����BaCO3��������ȷ����û��BaSO4����C��ȷ��
D���������Ϸ�����֪��ԭ��Һ��һ������SiO32-��AlO2-��Na+����CO32-��SO32-������һ�֣���ȷ���Ƿ���BaSO4����D����
��ѡC��
����ʵ��ڼ�̼����狀���г����������壬˵����Һ����Al3+����ԭ��Һ��AlO2-��HBr��Ӧ��ת��ΪAl3+������ΪAl��OH��3������ΪCO2��
����ʵ��ۣ�������̼�������Ba��OH��2��Ӧ����������NH3������һ����BaCO3��������ȷ����û��BaSO4��
A���������Ϸ�����֪���������Ϊ������̼�Ͷ�������Ļ�����A����
B��ʵ��ڼ�̼����狀���г����������壬˵����Һ����Al3+����ԭ��Һ��AlO2-��HBr��Ӧ��ת��ΪAl3+������ΪAl��OH��3������ΪCO2��ԭ��Һ�д��ڵ���AlO2-����B����
C����ʵ��ۣ�������̼�������Ba��OH��2��Ӧ����������NH3��������һ����BaCO3��������ȷ����û��BaSO4����C��ȷ��
D���������Ϸ�����֪��ԭ��Һ��һ������SiO32-��AlO2-��Na+����CO32-��SO32-������һ�֣���ȷ���Ƿ���BaSO4����D����
��ѡC��
����������Ϊ���ƶ��⣬�����˳����������ӵļ��鷽������Ŀ�Ѷ��еȣ�ע�����ճ������ӵ����ʼ����鷽������ȷijЩ���ӵ�������Ӧ�ǽ���ؼ��������и�����Һ�������ж������ӵĴ���Ϊ�״��㣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
