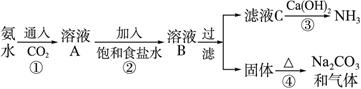
��Ŀ����
��֪��KClO3+6HCl=KCl+3Cl2��+3H2O���÷�Ӧ�е�������Ϊ______����ԭ��Ϊ______���μӷ�Ӧ��HCl�뱻������HCl��������______����ȡ250ml12mol/L��������һ������KClO3�����ַ�Ӧ��÷ų����������Ϊ6.72������״�������˹���ת�Ƶĵ�����Ϊ______����������Һ����仯����Ӧ����Һ�е���������ʵ���Ũ��Ϊ______mol/L��ȡ100ml����Һ����______��NaOH����ǡ�÷�Ӧ��ȫ��
��ӦKClO3+6HCl=KCl+3Cl2��+3H2O�У�ֻ��ClԪ�ػ��ϼ۷����仯������KClO3Ϊ��������HClΪ��ԭ����HCl�ڷ�Ӧ�б���Ϊ���Ժͻ�ԭ�ԣ��ɷ���ʽ��֪����6molHCl�μӷ�Ӧʱ����5mol����������μӷ�Ӧ��HCl�뱻������HCl��������6��5��
�ɷ���ʽ��֪������3molCl2ʱ��ת��5mol���ӣ���ȡ250ml12mol/L��������һ������KClO3�����ַ�Ӧ��÷ų����������Ϊ6.72������״������n��Cl2��=
=0.3mol����ת��0.5mol���ӣ�
��˹���ת�Ƶĵ�����Ϊ0.5mol��6.02��1023/mol=3.01��1023��
��Ӧ��ʣ��HCl�����ʵ���Ϊ0.25L��12mol/L-0.6mol=2.4mol��
��Ӧ������ʵ���Ũ��Ϊ
=9.6mol/L��
100mLʱ��n��HCl��=0.1L��9.6mol/L=0.96mol��
��Ҫ0.96molNaOH��Һ��m��NaOH��=0.96mol��40g/mol=38.4g��
�ʴ�Ϊ��KClO3��HCl��6��5��3.01��1023��9.6��38.4��
�ɷ���ʽ��֪������3molCl2ʱ��ת��5mol���ӣ���ȡ250ml12mol/L��������һ������KClO3�����ַ�Ӧ��÷ų����������Ϊ6.72������״������n��Cl2��=
| 6.72L |
| 22.4L/mol |
��˹���ת�Ƶĵ�����Ϊ0.5mol��6.02��1023/mol=3.01��1023��
��Ӧ��ʣ��HCl�����ʵ���Ϊ0.25L��12mol/L-0.6mol=2.4mol��
��Ӧ������ʵ���Ũ��Ϊ
| 2.4mol |
| 0.25L |
100mLʱ��n��HCl��=0.1L��9.6mol/L=0.96mol��
��Ҫ0.96molNaOH��Һ��m��NaOH��=0.96mol��40g/mol=38.4g��
�ʴ�Ϊ��KClO3��HCl��6��5��3.01��1023��9.6��38.4��

��ϰ��ϵ�д�
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д�
�����Ŀ
 Na2S+2CO2��
Na2S+2CO2��