��Ŀ����
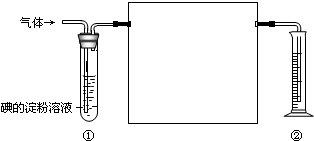 һ��������к���SO2��N2��O2����ѧ��ȤС���ͬѧ���ⶨ����SO2�������������ͼ�������ڲⶨ��ʹ�õIJ���ʵ��װ�ã�
һ��������к���SO2��N2��O2����ѧ��ȤС���ͬѧ���ⶨ����SO2�������������ͼ�������ڲⶨ��ʹ�õIJ���ʵ��װ�ã���1���ڷ����л�����ȱʵ��װ�ò����������Լ���
��2����װ�â��г���
��3��װ�â��з�����Ӧ�����ӷ���ʽΪ
��4������Ϊ�����Լ��У��������������Թ��е�ĵ�����Һ����
A������KMnO4��Һ B��NaOH��Һ C����ˮ D����ˮ
��5��������Һ��Ũ��Ϊ0.05mol/L�����Ϊ10mL���ռ�����N2��O2�����Ϊ148.8mL�����ۻ�Ϊ��״���µ��������SO2�������Ϊ
��������1��ʵ��ͨ��װ�âٺ����������գ��ٲⶨʣ����������������ȷ�������ж��������������ʲ�ȡ��Һ��ķ����ⶨʣ������������
��2����Ӧ������Һ��ɫ��ʧ��û�м�ʱֹͣͨ������ⷴӦʱ����������������²ⶨ��ʣ����������ƫ���²�õ�SO2�ĺ���ƫ�ͣ�
ͨ����������ʹ��죬������������������ȫ���գ����ֶ��������ų������²ⶨ��ʣ����������ƫ���²�õ�SO2�ĺ���ƫ�ͣ�
��3����������͵ⵥ�ʷ�Ӧ���ɵ⻯������ᣬ�ݴ�д�����ӷ���ʽ��
��4��װ�âٵ������Ƿ�Ӧ���ն�������װ���ڵ���Һ����������������ɷ֣����ѡ������ʵ������жϣ�
��5����Һ��ɫ�պ���ȥ˵���ⵥ��ǡ�÷�Ӧ�Ͷ�������Ӧ����ϻ�ѧ����ʽ����õ������������ʵ������ռ�����N2��O2�����Ϊ148.8mL�������ʵ������õ����������������������������ʵ����������㣮
��2����Ӧ������Һ��ɫ��ʧ��û�м�ʱֹͣͨ������ⷴӦʱ����������������²ⶨ��ʣ����������ƫ���²�õ�SO2�ĺ���ƫ�ͣ�
ͨ����������ʹ��죬������������������ȫ���գ����ֶ��������ų������²ⶨ��ʣ����������ƫ���²�õ�SO2�ĺ���ƫ�ͣ�
��3����������͵ⵥ�ʷ�Ӧ���ɵ⻯������ᣬ�ݴ�д�����ӷ���ʽ��
��4��װ�âٵ������Ƿ�Ӧ���ն�������װ���ڵ���Һ����������������ɷ֣����ѡ������ʵ������жϣ�
��5����Һ��ɫ�պ���ȥ˵���ⵥ��ǡ�÷�Ӧ�Ͷ�������Ӧ����ϻ�ѧ����ʽ����õ������������ʵ������ռ�����N2��O2�����Ϊ148.8mL�������ʵ������õ����������������������������ʵ����������㣮
����⣺��1��ʵ��ͨ��װ�âٺ����������գ��ٲⶨʣ����������������ȷ�������ж��������������ʲ�ȡ��Һ��ķ����ⶨʣ���������������ڵ���������������ˮ���ʿ�������ˮ���ⶨʣ�����������װ��ͼΪ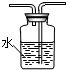 ��
��
�ʴ�Ϊ�� ��
��
��2����Һ��ɫ�պ���ȥ��Ӧ��ʱֹͣͨ��������Ӧ������Һ��ɫ��ʧ��û�м�ʱֹͣͨ������ⷴӦʱ����������������²ⶨ��ʣ����������ƫ�ⶨ�Ķ�����������ƫС�����²�õ�SO2�ĺ���ƫ�ͣ�
ͨ����������ʹ��죬������������������ȫ���գ����ֶ��������ų������²ⶨ��ʣ����������ƫ�ⶨ�Ķ�����������ƫС�����²�õ�SO2�ĺ���ƫ�ͣ�
�ʴ�Ϊ����Һ��ɫ�պ���ȥ��ƫ�ͣ�ƫ�ͣ�
��3����������͵ⵥ�ʷ�Ӧ���ɵ⻯������ᣬ�ݴ�д��װ�â��з�����Ӧ�����ӷ���ʽΪSO2+I2+2H2O=4H++SO42-+2I-��
�ʴ�Ϊ��SO2+I2+2H2O=4H++SO42-+2I-��
��4��װ�âٵ������Ƿ�Ӧ���ն�������װ���ڵ���Һ����������������ɷ֣�
A�����������Һ�����������������������壬��Ӱ��ʣ����������IJⶨ����A��ȷ��
B������������Һ�������ն������������ж��Ƿ�������ȫ���ⶨ��SO2�ĺ������������B����
C����ˮ��Һ�������ն����������ն��������������ԣ����Դ��棬��C��ȷ��
D����ˮ��Һ���ն��������Ƿ���ȫ�������жϣ���D����
��ѡ��AC��
��5��������Һ��Ũ��Ϊ0.05mol/L�����Ϊ10mL�����ʵ���=0.05mol/L��0.010L=0.0005mol�������������ʵ���Ϊ0.0005mol����״�������=0.0005mol��22.4L/mol=0.0112L���ռ�����N2��O2�����Ϊ148.8mL�����ۻ�Ϊ��״���µ��������SO2�������=
��100%=7%��
�ʴ�Ϊ��7%��
 ��
���ʴ�Ϊ��
 ��
����2����Һ��ɫ�պ���ȥ��Ӧ��ʱֹͣͨ��������Ӧ������Һ��ɫ��ʧ��û�м�ʱֹͣͨ������ⷴӦʱ����������������²ⶨ��ʣ����������ƫ�ⶨ�Ķ�����������ƫС�����²�õ�SO2�ĺ���ƫ�ͣ�
ͨ����������ʹ��죬������������������ȫ���գ����ֶ��������ų������²ⶨ��ʣ����������ƫ�ⶨ�Ķ�����������ƫС�����²�õ�SO2�ĺ���ƫ�ͣ�
�ʴ�Ϊ����Һ��ɫ�պ���ȥ��ƫ�ͣ�ƫ�ͣ�
��3����������͵ⵥ�ʷ�Ӧ���ɵ⻯������ᣬ�ݴ�д��װ�â��з�����Ӧ�����ӷ���ʽΪSO2+I2+2H2O=4H++SO42-+2I-��
�ʴ�Ϊ��SO2+I2+2H2O=4H++SO42-+2I-��
��4��װ�âٵ������Ƿ�Ӧ���ն�������װ���ڵ���Һ����������������ɷ֣�
A�����������Һ�����������������������壬��Ӱ��ʣ����������IJⶨ����A��ȷ��
B������������Һ�������ն������������ж��Ƿ�������ȫ���ⶨ��SO2�ĺ������������B����
C����ˮ��Һ�������ն����������ն��������������ԣ����Դ��棬��C��ȷ��
D����ˮ��Һ���ն��������Ƿ���ȫ�������жϣ���D����
��ѡ��AC��
��5��������Һ��Ũ��Ϊ0.05mol/L�����Ϊ10mL�����ʵ���=0.05mol/L��0.010L=0.0005mol�������������ʵ���Ϊ0.0005mol����״�������=0.0005mol��22.4L/mol=0.0112L���ռ�����N2��O2�����Ϊ148.8mL�����ۻ�Ϊ��״���µ��������SO2�������=
| 0.0112L |
| 0.0112L+0.1488L |
�ʴ�Ϊ��7%��
���������⿼��ѧ����ʵ��ԭ����װ�õ����⣬��ʵ�鷽�������ۡ���ͼ��������ѧ����ȣ��Ѷ��еȣ��Ƕ���ѧ֪ʶ���ۺ����ã���Ҫѧ���߱���ʵ�Ļ���֪ʶ��������⡢���������������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ