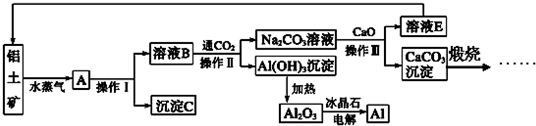
��Ŀ����
��ش��������⣺��1�����л��������۵���ߵ���
A��NaCl B��KBr C��NaF D��KI
��2����̬����Cr��ԭ�ӵ���Χ�����Ų�ʽ��
��3����ˮ�д��ڶ�����ʽ������������롰����������ˮ������������ص�����ɱ�ʾΪ��
a��������ˮ���� b����HBr�ķе����HCl c�����ǻ�����ȩ���۵���ڶ��ǻ�����ȩ
d��ˮ���ӱ���������ȶ� e�������Ҵ��е��ܽ�Ƚϴ��������Ҵ��ӵ�ˮ����ȡ��
��4���������ʵľ����к��������ӵ���
A��K B��H2O2 C��CH3NH2 D��HCOONa E��F2C=CF2 F��CO2 G��C2HCl2Br
��5�����и���ıȽ�����ȷ����
A����һ�����ܣ�Mg��Al B���縺�ԣ�P��Ge C���ȶ��ԣ�AsH3��H2S D�������ԣ�20X��30Y��
��������1�����Ӿ��������Ӱ뾶ԽС�����Ӽ�Խǿ���۵�Խ�ߣ�
��2��CrΪ24��Ԫ�أ����ݵ����Ų�ʽ����������ϵĵ��Ӵ��ڰ�����ȫ��ʱ������ͣ�
��3����ˮ��O��H��N��H֮���γ����������������������ʵ�Ӱ�������
��4�����Ӿ���ͽ��������к��������ӣ�����˫���������ķ����Цм���ͬ�ַǽ���Ԫ���γɷǼ��Լ�����ͬ�ǽ����γɼ��Լ���
��5��A��Mg����������Ϊȫ��״̬���ṹ�ȶ�����һ�����ܱ�Al��
B���ǽ�����Խǿ�縺��Խǿ��
C���ǽ�����Խǿ��Ӧ���⻯��Խ�ȶ���
D������Ԫ�������ڱ��е�λ���жϣ�
��2��CrΪ24��Ԫ�أ����ݵ����Ų�ʽ����������ϵĵ��Ӵ��ڰ�����ȫ��ʱ������ͣ�
��3����ˮ��O��H��N��H֮���γ����������������������ʵ�Ӱ�������
��4�����Ӿ���ͽ��������к��������ӣ�����˫���������ķ����Цм���ͬ�ַǽ���Ԫ���γɷǼ��Լ�����ͬ�ǽ����γɼ��Լ���
��5��A��Mg����������Ϊȫ��״̬���ṹ�ȶ�����һ�����ܱ�Al��
B���ǽ�����Խǿ�縺��Խǿ��
C���ǽ�����Խǿ��Ӧ���⻯��Խ�ȶ���
D������Ԫ�������ڱ��е�λ���жϣ�
����⣺��1�����Ӿ��������Ӱ뾶ԽС�����Ӽ�Խǿ���۵�Խ�ߣ�4��������NaF �����Ӱ뾶��С������NaF���۵���ߣ��ʴ�Ϊ��C��
��2��CrΪ24��Ԫ�أ�������Ų�ʽ1s22s22p63s23p63d54s1�����Ի�̬����Cr��ԭ�ӵ���Χ�����Ų�ʽ��3d54s1������3d��4s����ϵĵ��Ӵ��ڰ���״̬����������ϵͣ��ʴ�Ϊ��3d54s1��3d��4s����ϵĵ��Ӿ�Ϊ�����״̬��
��3����ˮ��O��H��N��H֮���γ������O-H��N��N-H��O��
a��������ˮ���ܣ�����Ϊ�����е��ǻ���ˮ���Ӽ����γ����������������йأ���a��ȷ��
b��HBr��Է���������HCl�Ĵ�����HBr�ķе����HCl��������أ���b����
c�����ǻ�����ȩ���γɷ�������������ǻ�����ȩ���γɷ��Ӽ�������������ǻ�����ȩ���۵���ڶ��ǻ�����ȩ��������йأ���c��ȷ��
d�����ӵ��ȶ���������еĹ��ۼ��йأ�������أ���d����
e���Ҵ���ˮ���γɷ��Ӽ�����������Ҵ���ˮ���ܣ��������Ҵ��ӵ�ˮ����ȡ�⣬������йأ���e��ȷ��
�ʴ�Ϊ��O-H��N��N-H��O��ace��
��4�����Ӿ���ͽ��������к��������ӣ����������ӵ���AD������˫���������ķ����Цм������Է����д��ڦм�����DEF���Ⱥ����Լ��ֺ��Ǽ��Լ�����BG��
�ʴ�Ϊ��AD��DEF��BG��
��5��A��Mg����������Ϊȫ��״̬���ṹ�ȶ�����һ�����ܱ�Al����һ�����ܣ�Mg��Al����A��ȷ��
B���ǽ�����Խǿ�縺��Խǿ���ǽ����ԣ�P��Ge�����Ե縺�ԣ�P��Ge����B��ȷ��
C���ǽ�����Խǿ��Ӧ���⻯��Խ�ȶ����ǽ����ԣ�S��As�������ȶ��ԣ�AsH3��H2S����C����
D��20XΪCaԪ�أ�30YΪZnԪ�أ�����ͬ���������ڣ�CaԪ����ǰ�棬����Ca������ǿ����D����
�ʴ�Ϊ��AB��
��2��CrΪ24��Ԫ�أ�������Ų�ʽ1s22s22p63s23p63d54s1�����Ի�̬����Cr��ԭ�ӵ���Χ�����Ų�ʽ��3d54s1������3d��4s����ϵĵ��Ӵ��ڰ���״̬����������ϵͣ��ʴ�Ϊ��3d54s1��3d��4s����ϵĵ��Ӿ�Ϊ�����״̬��
��3����ˮ��O��H��N��H֮���γ������O-H��N��N-H��O��
a��������ˮ���ܣ�����Ϊ�����е��ǻ���ˮ���Ӽ����γ����������������йأ���a��ȷ��
b��HBr��Է���������HCl�Ĵ�����HBr�ķе����HCl��������أ���b����
c�����ǻ�����ȩ���γɷ�������������ǻ�����ȩ���γɷ��Ӽ�������������ǻ�����ȩ���۵���ڶ��ǻ�����ȩ��������йأ���c��ȷ��
d�����ӵ��ȶ���������еĹ��ۼ��йأ�������أ���d����
e���Ҵ���ˮ���γɷ��Ӽ�����������Ҵ���ˮ���ܣ��������Ҵ��ӵ�ˮ����ȡ�⣬������йأ���e��ȷ��
�ʴ�Ϊ��O-H��N��N-H��O��ace��
��4�����Ӿ���ͽ��������к��������ӣ����������ӵ���AD������˫���������ķ����Цм������Է����д��ڦм�����DEF���Ⱥ����Լ��ֺ��Ǽ��Լ�����BG��
�ʴ�Ϊ��AD��DEF��BG��
��5��A��Mg����������Ϊȫ��״̬���ṹ�ȶ�����һ�����ܱ�Al����һ�����ܣ�Mg��Al����A��ȷ��
B���ǽ�����Խǿ�縺��Խǿ���ǽ����ԣ�P��Ge�����Ե縺�ԣ�P��Ge����B��ȷ��
C���ǽ�����Խǿ��Ӧ���⻯��Խ�ȶ����ǽ����ԣ�S��As�������ȶ��ԣ�AsH3��H2S����C����
D��20XΪCaԪ�أ�30YΪZnԪ�أ�����ͬ���������ڣ�CaԪ����ǰ�棬����Ca������ǿ����D����
�ʴ�Ϊ��AB��
�����������ۺϿ�����ԭ�ӵĽṹ�����ʼ�Ԫ�������ɣ��漰�����Ų�ʽ�������Էǽ����ԵıȽϡ�����������۵�ıȽϵȣ������֪ʶ��϶࣬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
�����Ŀ
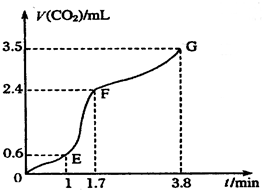 ��������̼��ƹ����ϡ���ᷴӦ��ȡCO2���壬��ش��������⣺
��������̼��ƹ����ϡ���ᷴӦ��ȡCO2���壬��ش��������⣺