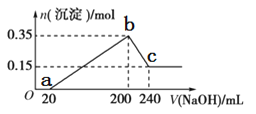
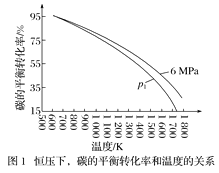
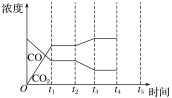
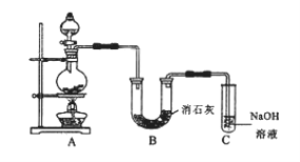
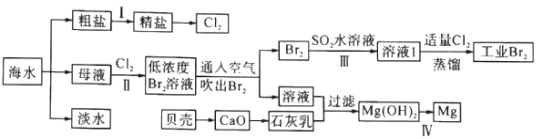
��Ŀ����
����Ŀ��ϩ������Ҫ���л�����ԭ�ϣ����л��ϳ������Ź㷺��Ӧ�á�
I.��֪��ϩ�����ֽⷴӦ��ָ�ڴ��������£�ʵ��![]() �����Ż�λ�ķ�Ӧ��2CH2=CHCH3
�����Ż�λ�ķ�Ӧ��2CH2=CHCH3![]() CH3CH=CHCH3+CH2=CH2�����ⶨ
CH3CH=CHCH3+CH2=CH2�����ⶨ![]() ��һ��ͬ���칹��M�ĺ˴Ź��������������壬�����֮��Ϊ1��3��
��һ��ͬ���칹��M�ĺ˴Ź��������������壬�����֮��Ϊ1��3��![]() ��ͼΪM�ϳ�Q������ͼ��
��ͼΪM�ϳ�Q������ͼ��
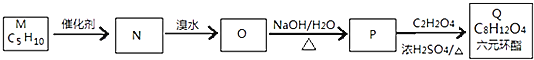
![]() ������Ϊ ______ ��
������Ϊ ______ ��
![]() �Ļ�ѧ��Ӧ����Ϊ ______ ��
�Ļ�ѧ��Ӧ����Ϊ ______ ��
![]() �
д��![]() �Ļ�ѧ����ʽ ______ ��
�Ļ�ѧ����ʽ ______ ��
![]() ���
�л���![]() �㷺�����㾫�ĵ������ʵ���ҵĿƼ���Ա����M��һ��ͬ���칹�壬ͨ���������̺ϳ��л���E��
�㷺�����㾫�ĵ������ʵ���ҵĿƼ���Ա����M��һ��ͬ���칹�壬ͨ���������̺ϳ��л���E��
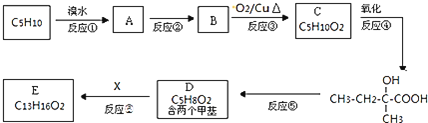
![]() �Ļ�ѧ����ʽΪ ______ ��
�Ļ�ѧ����ʽΪ ______ ��
![]() ��֪X�Ļ���ֻ��һ��ȡ��������ȡ������֧������E�Ľṹ��ʽΪ ______ ��
��֪X�Ļ���ֻ��һ��ȡ��������ȡ������֧������E�Ľṹ��ʽΪ ______ ��
![]() д����������������X��ͬ���칹��Ľṹ��ʽ�� ______
д����������������X��ͬ���칹��Ľṹ��ʽ�� ______
![]() ��
��![]() ��Һ����ɫ
��Һ����ɫ![]() �����ϵ�һ��ȡ����ֻ�����֡�
�����ϵ�һ��ȡ����ֻ�����֡�
���𰸡�![]() ��
��![]() ��ϩ ˮ�ⷴӦ
��ϩ ˮ�ⷴӦ![]() ��ȡ����Ӧ
��ȡ����Ӧ![]()
 +HOOC-COOH
+HOOC-COOH![]()
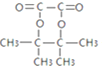 +2H2O 2
+2H2O 2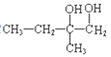 +O2
+O2![]() 2
2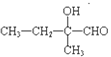 +2H2O
+2H2O ![]()
��������
![]() ���ⶨ
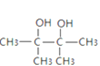
���ⶨ![]() ��һ��ͬ���칹��M�ĺ˴Ź��������������壬�����֮��Ϊ1��3��6����M�Ľṹ��ʽΪ��
��һ��ͬ���칹��M�ĺ˴Ź��������������壬�����֮��Ϊ1��3��6����M�Ľṹ��ʽΪ��![]() ��P���Ҷ��ᷢ��������Ӧ����Q��Q����Ԫ��������P���Ҷ�����O���������Ƶ�ˮ��Һ����ȡ����Ӧ�����Ҷ�����N���巢���ӳɷ�Ӧ��������飬����̼ԭ���غ�֪��N����ϩ��O��1��
��P���Ҷ��ᷢ��������Ӧ����Q��Q����Ԫ��������P���Ҷ�����O���������Ƶ�ˮ��Һ����ȡ����Ӧ�����Ҷ�����N���巢���ӳɷ�Ӧ��������飬����̼ԭ���غ�֪��N����ϩ��O��1��![]() �������飻
�������飻
![]() �Ľṹ��ʽΪ��
�Ľṹ��ʽΪ��![]() ����M�������ǣ�
����M�������ǣ�![]() ��
��![]() ��ϩ��
��ϩ��
�ʴ�Ϊ��![]() ��
��![]() ��ϩ��
��ϩ��
![]() ��
��![]() �������鷢��ˮ�ⷴӦ
�������鷢��ˮ�ⷴӦ![]() ��ȡ����Ӧ
��ȡ����Ӧ![]() �����Ҷ������ʴ�Ϊ��ˮ�ⷴӦ
�����Ҷ������ʴ�Ϊ��ˮ�ⷴӦ![]() ��ȡ����Ӧ
��ȡ����Ӧ![]() ��
��
![]() ��Ũ���������������������·���������Ӧ����Ӧ����ʽΪ��
��Ũ���������������������·���������Ӧ����Ӧ����ʽΪ��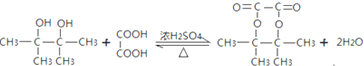 ��
��
�ʴ�Ϊ�� ��
��
![]() ����
����![]() ��
��![]() �ǻ�
�ǻ�![]() ����Ľṹ��ʽ���D�ķ���ʽ֪��D�Ľṹ��ʽΪ��
����Ľṹ��ʽ���D�ķ���ʽ֪��D�Ľṹ��ʽΪ��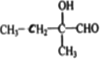 ��B��������C����B�Ľṹ��ʽΪ��
��B��������C����B�Ľṹ��ʽΪ��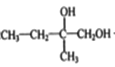 ��ϩ�����巢���ӳɷ�Ӧ����A��A���������Ƶ�ˮ��Һ����ȡ����Ӧ����B������B�Ľṹ��ʽ֪��A�Ľṹ��ʽΪ��
��ϩ�����巢���ӳɷ�Ӧ����A��A���������Ƶ�ˮ��Һ����ȡ����Ӧ����B������B�Ľṹ��ʽ֪��A�Ľṹ��ʽΪ��![]() �����ϩ���Ľṹ��ʽΪ��
�����ϩ���Ľṹ��ʽΪ��![]() ��
��![]() ��
��![]() �ǻ�
�ǻ�![]() ���ᷢ����ȥ��Ӧ����D����������֪��D�Ľṹ��ʽΪ��
���ᷢ����ȥ��Ӧ����D����������֪��D�Ľṹ��ʽΪ��![]() ��D��X��Ӧ����E��X�Ļ���ֻ��һ��ȡ��������ȡ������֧��������X�Ľṹ��ʽΪ��
��D��X��Ӧ����E��X�Ļ���ֻ��һ��ȡ��������ȡ������֧��������X�Ľṹ��ʽΪ��![]() ��E�Ľṹ��ʽΪ��
��E�Ľṹ��ʽΪ��![]() ��
��
![]() ��ͭ�����������������±�������������C����Ӧ����ʽΪ��
��ͭ�����������������±�������������C����Ӧ����ʽΪ��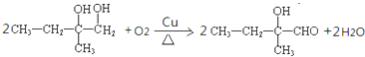 ��
��
�ʴ�Ϊ��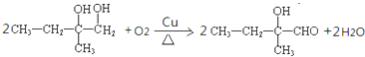 ��
��
![]() ͨ�����Ϸ���֪��E�Ľṹ��ʽΪ��
ͨ�����Ϸ���֪��E�Ľṹ��ʽΪ��![]() ��
��
�ʴ�Ϊ��![]() ��
��
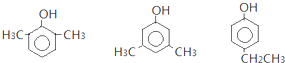
![]() ��ͬ���칹�����������������
��ͬ���칹�����������������![]() ��Һ����ɫ˵�����з��ǻ��������ϵ�һ��ȡ����ֻ�����֣�
��Һ����ɫ˵�����з��ǻ��������ϵ�һ��ȡ����ֻ�����֣�
���Է��������Ľṹ��ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��