��Ŀ����
��14�֣���ͳ�Ķ�����ѧʵ���ܵ������ֶε���Լ���о���Χ��խ����ȷ�Ȳ��ߡ�����DISϵͳ�������ֻ���Ϣϵͳ���ɴ����������ݲɼ����ͼ������ɣ��ķ�����ʹ�ã���һ�������˺ܴ�̶ȵĸ��ơ�ijѧϰС�������кͷ�Ӧԭ����DISϵͳ�ⶨij��ˮ�����ʵ���Ũ�ȣ��Բ�����Һ������������ �ϵζ��յ㡣ʵ�鲽�����£�
�ϵζ��յ㡣ʵ�鲽�����£�
���� �����������ƣ���ȡ10.00mL��ˮ��Ʒ���� �����������ƣ�����һ��������ˮϡ�Ͳ� ��ת�ƣ���ϴ��Һ����100mL �����������ƣ��У��� �����������ƣ�������ˮ���ݡ����Ⱥ�������Һװ���Լ�ƿ���á�
����ȡ20.00mL������Һ����ྻ������ƿ�У����Ӻ�DISϵͳ�� �����ƿ�к�����������ˮ���Ƿ��Ӱ�������� ����ǡ�������������ȷ��������
�����ƿ�к�����������ˮ���Ƿ��Ӱ�������� ����ǡ�������������ȷ��������
������ ��
������ƿ�еμ�0.1000mol/L�����ᣬ�������Ļ����ʾ����Һ����������������������ϵ�� ����ͼ��
����ͼ��

�ٵζ���ʢ������ǰ��Ҫ�� ��Ȼ��������ˮ��ϴ2��3�Σ����� ��Ȼ�����ʢ�����ᡣ�ζ����м������ᡢ�ž����첿�ֵ����ݺ�Һ�����͵�Ӧ���ڵζ��ܵ� ��
�ڰ�ˮ�����ᷴӦ�����ӷ���ʽΪ ��
�۸ð�ˮ��Ʒ�����ʵ���Ũ��Ϊ mol?L-1��
����һѧϰС������Ϊ������������Դ���һ��������Ϊ���ɵ�NH4Cl��ǿ�������Σ��ᷢ��ˮ���ʹNH+ 4Ũ���½���������ǡ����ȫ��ӦʱNH+ 4Ũ�Ȳ������ֵ����Һ�����ԾͲ��������ֵ��
������ΪѧϰС���ҵĽ����Ƿ���ȷ�� �����ȷ�� ��������ȷ����
��������ȷ����
�ڵ���ҺpH=7ʱ����Һ�и�����Ũ�ȴ�С�Ĺ�ϵ�� ��
 �ϵζ��յ㡣ʵ�鲽�����£�
�ϵζ��յ㡣ʵ�鲽�����£����� �����������ƣ���ȡ10.00mL��ˮ��Ʒ���� �����������ƣ�����һ��������ˮϡ�Ͳ� ��ת�ƣ���ϴ��Һ����100mL �����������ƣ��У��� �����������ƣ�������ˮ���ݡ����Ⱥ�������Һװ���Լ�ƿ���á�
����ȡ20.00mL������Һ����ྻ������ƿ�У����Ӻ�DISϵͳ��
 �����ƿ�к�����������ˮ���Ƿ��Ӱ�������� ����ǡ�������������ȷ��������
�����ƿ�к�����������ˮ���Ƿ��Ӱ�������� ����ǡ�������������ȷ�������������� ��
������ƿ�еμ�0.1000mol/L�����ᣬ�������Ļ����ʾ����Һ����������������������ϵ��
 ����ͼ��
����ͼ��
�ٵζ���ʢ������ǰ��Ҫ�� ��Ȼ��������ˮ��ϴ2��3�Σ����� ��Ȼ�����ʢ�����ᡣ�ζ����м������ᡢ�ž����첿�ֵ����ݺ�Һ�����͵�Ӧ���ڵζ��ܵ� ��
�ڰ�ˮ�����ᷴӦ�����ӷ���ʽΪ ��
�۸ð�ˮ��Ʒ�����ʵ���Ũ��Ϊ mol?L-1��
����һѧϰС������Ϊ������������Դ���һ��������Ϊ���ɵ�NH4Cl��ǿ�������Σ��ᷢ��ˮ���ʹNH+ 4Ũ���½���������ǡ����ȫ��ӦʱNH+ 4Ũ�Ȳ������ֵ����Һ�����ԾͲ��������ֵ��
������ΪѧϰС���ҵĽ����Ƿ���ȷ�� �����ȷ��
 ��������ȷ����
��������ȷ�����ڵ���ҺpH=7ʱ����Һ�и�����Ũ�ȴ�С�Ĺ�ϵ�� ��
�ż�ʽ�ζ��ܣ�����Һ�ܣ����ձ�����С�ձ�������ȴ������ƿ����ͷ�ιܣ�
�Ʒ�����������ˮϡ�ͣ�����ı䰱ˮ���ʵ����ʵ�����Ҳ�Ͳ���ı��кͷ�Ӧ����������ʵ�����
�Ǣټ����ʽ�ζ����Ƿ�©ˮ����Ҫʢ�ŵ�������ϴ2��3�Σ���0���̶Ȼ�0���̶����£�
�� NH3��H2O + H+ = NH+ 4 + H2O��
�� 1mol/L��
�Ȣٲ���ȷ��
�� c(Cl-)=c(NH+ 4)��c(H+)=c(OH-)
�Ʒ�����������ˮϡ�ͣ�����ı䰱ˮ���ʵ����ʵ�����Ҳ�Ͳ���ı��кͷ�Ӧ����������ʵ�����
�Ǣټ����ʽ�ζ����Ƿ�©ˮ����Ҫʢ�ŵ�������ϴ2��3�Σ���0���̶Ȼ�0���̶����£�
�� NH3��H2O + H+ = NH+ 4 + H2O��
�� 1mol/L��
�Ȣٲ���ȷ��
�� c(Cl-)=c(NH+ 4)��c(H+)=c(OH-)
��

��ϰ��ϵ�д�
 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�
�����Ŀ
 ��δ�ô���Һ��ϴ
��δ�ô���Һ��ϴ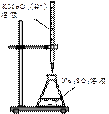


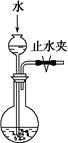 ���ر�ֹˮ�У�
���ر�ֹˮ�У�