��Ŀ����
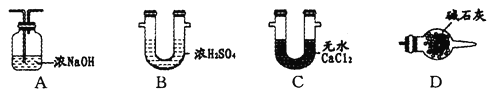
����Ŀ����ͼΪʵ������ȡ����������װ�á���ش�

��1��Ũ�����������______________________��
��2��װ���е����θ���ܳ������������⣬������һ��Ҫ������_______________��
��3�������йظ�ʵ���˵���У���ȷ����_____________��
A����A�Թ��м����ʯ���������Ƿ�ֹ����ʱҺ�屩��
B���Թ�B��ʢ�ŵ���ҺΪ����̼������Һ�����Գ�ȥ�����л��е�����
C������������һ����ɫ�����ܶȱ�ˮ�����״Һ��
D����ԭ��ΪCH3COOH��CH3CH218OH�������������в���18O
��4����ͼ�Ƕ��Թ�B���ռ����Ļ������з���õ�����������������Ҵ���ʵ���������ͼ��

������ʵ������У�����˵����ȷ����___________(��ѡ��)��
A������1���� B������2����
C��Ϊ�õ��������������������ѡ�ü�ʯ��������� D���Լ�C��Ϊ����
��5��д��ʵ�����Ʊ����������Ļ�ѧ����ʽ______________________________��
���𰸡���������ˮ�� ��ֹ���� AB B CH3COOH+C2H5OH ![]() CH3COOC2H5+H2O
CH3COOC2H5+H2O
��������
(1)������ӦΪ���淴Ӧ����Ũ���������ˮ�ԣ�ˮ�ļ���������ƽ�������ƶ���
(2)�Ҵ�������������ˮ��������������
(3)A��Һ���������Ҫ��ֹ���У�B��̼������Һ�������ᷴӦ��C������������һ����ɫ�����ܶȱ�ˮС����״Һ�壻D������CH3CH218OH��CH3COOH����������Ӧ��ԭ���ǣ������ǻ������⣻
(4)�����̿�֪��̼������Һ�����������ֲ㣬����1Ϊ��Һ��Y�к������ơ��Ҵ���̼���ƣ�����2Ϊ�������WΪ�Ҵ���Z�к������ơ�̼���ƣ����Լ�CΪ���ᣬ����3Ϊ�������õ����
(5)�Ҵ���������Ũ���������¼�����������������ˮ��
(1)������Ӧ��Ũ�������Ҫ�����Ǵ�������ˮ����
(2)װ���е����θ���ܳ������������⣬�����Է�ֹҺ�嵹����
(3)A��Һ���������Ҫ��ֹ���У���A�Թ��м����ʯ���������Ƿ�ֹ����ʱҺ�屩�У���A��ȷ��B��̼������Һ�������ᷴӦ�����Ա���̼������Һ���Գ�ȥ�����л��е����ᣬ��B��ȷ��C���Ҵ������ᷴӦ���ɵ���������������������һ����ɫ�����ܶȱ�ˮС����״Һ�壬��C����D������CH3CH218OH��CH3COOH����������Ӧ��ԭ���ǣ������ǻ������⣬���������к�18O����D���ʴ�ΪAB��
(4)�����̿�֪��̼������Һ�����������ֲ㣬����1Ϊ��Һ��Y�к������ơ��Ҵ���̼���ƣ�����2Ϊ�������WΪ�Ҵ���Z�к������ơ�̼���ƣ����Լ�CΪ���ᣬ����3Ϊ�������õ�����������������ڼ�����������ˮ�⣬�������������������ѡ�ü�ʯ������������ʴ�ΪB��
(5)�Ҵ���������Ũ���������¼�����������������ˮ��������Ӧ�Ļ�ѧ����ʽΪCH3COOH+C2H5OH ![]() CH3COOC2H5+H2O��
CH3COOC2H5+H2O��

 ����������������ϵ�д�
����������������ϵ�д�