��Ŀ����
�״�����Ҫ�Ļ�ѧ��ҵ����ԭ�Ϻ����Һ��ȼ�ϡ���ҵ�Ͽ�����CO��CO2������ȼ�ϼ״�����֪�״��Ʊ����йػ�ѧ��Ӧ�Լ��ڲ�ͬ�¶��µĻ�ѧ��Ӧƽ�ⳣ�����±���ʾ��
��ѧ��Ӧ | ƽ�ⳣ�� | �¶ȡ� | |
500 | 800 | ||
��2H2(g)+CO(g) | K1 | 2.5 | 0.15 |
��H2(g)+CO2(g) | K2 | 1.0 | 2.50 |
��3H2(g)+CO2(g) | K3 | ||
��1����Ӧ����________________������ȡ����ȡ�����Ӧ��
��2��ij�¶��·�Ӧ����H2��ƽ��ת���ʣ�a������ϵ��ѹǿ(P)�Ĺ�ϵ��������ͼ��ʾ����ƽ��״̬��A�䵽Bʱ��ƽ�ⳣ��K(A)_____________K(B)������������������������ݷ�Ӧ����ڿ��Ƶ���K1��K2��K3֮��Ĺ�ϵ����K3=_______����K1��K2��ʾ����

��3����3 L�ݻ��ɱ���ܱ������з�����Ӧ�ڣ���֪c(CO)�뷴Ӧʱ��t�仯���ߢ�������ͼ��ʾ������t0ʱ�̷ֱ�ı�һ�����������ߢ��Ϊ���ߢ�����ߢ�
�����ߢ��Ϊ���ߢ�ʱ���ı��������_____________________��
�����ߢ��Ϊ���ߢ�ʱ���ı��������_____________________��
��4��һ�ּ״�ȼ�ϵ�أ�ʹ�õĵ������Һ��2mol��L��1��KOH��Һ��
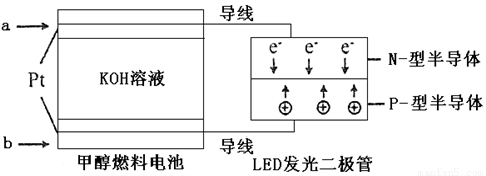
��д������(ͨ��)b����һ���ĵ缫��Ӧʽ_________________��
ÿ����6.4g�״�ת�Ƶĵ�����Ϊ_______________��
��5��һ�������¼״���һ����̼��Ӧ���Ժϳ����ᡣͨ��״���£���a mol/L�Ĵ�����b mol/LBa(OH)2��Һ�������Ϻ���Һ�У�2c(Ba2��)= c(CH3COO-)���ú�a��b�Ĵ���ʽ��ʾ�û����Һ�д���ĵ��볣��KaΪ________________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д� CH3OH(g)
CH3OH(g) H2O (g)+CO(g)
H2O (g)+CO(g) CH3OH(g)+H2O (g)
CH3OH(g)+H2O (g)

 4NO(g)+CO2(g)+2H2O(l) ��H=-662kJ/mol
4NO(g)+CO2(g)+2H2O(l) ��H=-662kJ/mol

 ��ZX��һ���������W��Yͬһ���塣����������ȷ���ǣ� ��
��ZX��һ���������W��Yͬһ���塣����������ȷ���ǣ� ��