��Ŀ����
���ҵĻ������÷����ܶ࣬���ú���Al2O3��SiO2������FeO xFe2O3�������Ʊ�Al2(S04)3
xFe2O3�������Ʊ�Al2(S04)3 18H2O�������������£�
18H2O�������������£�
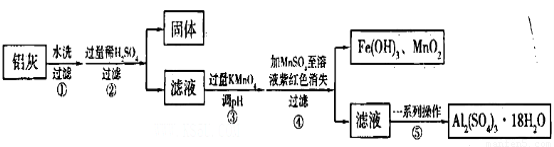
��ش��������⣺
��1���������ϡH2SO4�ܽ�Al2O3�����ӷ���ʽ��______________��
��2�������м��˵�KMnO4Ҳ����H2O2���棬����H2O2������Ӧ�Ļ�ѧ����ʽΪ_______________��
��3����֪��Ũ�Ⱦ�ΪO.1mol/L�Ľ��������ӣ������������������pH���±���
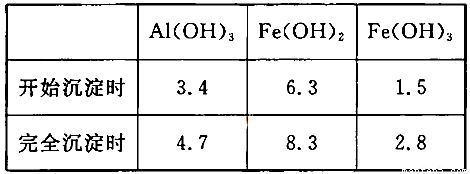
��������Ŀ����__________________________________________________________�����ڸ�Ũ���³�ȥ���Ļ��������pH�����Χ��___________��
��4����֪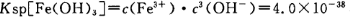 ��pH=2ʱ��Fe3����ʼ������Ũ��Ϊ_______________��
��pH=2ʱ��Fe3����ʼ������Ũ��Ϊ_______________��
��5��������������Ӧ�����ӷ���ʽΪ__________________________________________��Ϊ����֤�ò������ù�����ȷʵ����MnO2����ѡ�õ��Լ���_________��_________��
��6����������һϵ�в���"�����������в����õ���___________������ţ���
A��������? B������? C��������? D���ƾ���?? ? E��©��
��1��6H+ + Al2O3=2Al3+ + 3H2O ��2��H2O2 + 2FeSO4 + H2SO4 = Fe2(SO4)3 + 2H2O ����3���������������������������ӣ���ͨ������PHֵ������������ת��Ϊ��������������ȥ��2.8��3.4����4��4*10-2mol/L����5��3Mn2+ + 2MnO4- + 4OH- =5MnO2 + 2H2O ��Ũ����������������Һ����6�� B
��������
�����������1���������ϡH2SO4�ܽ�Al2O3�����ӷ���ʽ��6H+ + Al2O3=2Al3+ + 3H2O ����2�������м��˵�KMnO4Ҳ����H2O2���棬H2O2��ǿ�����Ѷ�������������Ϊ���������ӣ�������Ӧ�Ļ�ѧ����ʽΪH2O2 + 2FeSO4 + H2SO4 = Fe2(SO4)3 + 2H2O ����3����������ͼ��֪�ǽ������������������������ӣ���ͨ������PHֵ������������ת��Ϊ��������������ȥ��ȷ�������Ӳ�Ҫ�����������ʵ���pH��ΧΪ��2.8��3.4����4����֪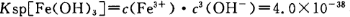 ��pH=2ʱ����c(H+)=0.01Mmol/L��c(OH-)=10-12Mmol/L������
��pH=2ʱ����c(H+)=0.01Mmol/L��c(OH-)=10-12Mmol/L������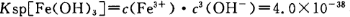 ��ʽ���ɵ�Fe3����ʼ������Ũ��Ϊ4*10-2mol/L����5�����ݲ����г��ֵ�������ɫ��ʧ��˵������������Ӳμ��˷�Ӧ����������Ӧ�����ӷ���ʽΪ3Mn2+ + 2MnO4- + 4OH- =5MnO2 + 2H2O ��Ϊ����֤�ò������ù�����ȷʵ����MnO2����ѡ�õ��Լ���_Ũ���Ტ���Ȼ����������Һ�����ݲ�������˵������6����������һϵ�в���"���У�����Ũ�������½ᾧ�����ˣ������õ��� B��������
��ʽ���ɵ�Fe3����ʼ������Ũ��Ϊ4*10-2mol/L����5�����ݲ����г��ֵ�������ɫ��ʧ��˵������������Ӳμ��˷�Ӧ����������Ӧ�����ӷ���ʽΪ3Mn2+ + 2MnO4- + 4OH- =5MnO2 + 2H2O ��Ϊ����֤�ò������ù�����ȷʵ����MnO2����ѡ�õ��Լ���_Ũ���Ტ���Ȼ����������Һ�����ݲ�������˵������6����������һϵ�в���"���У�����Ũ�������½ᾧ�����ˣ������õ��� B��������
���㣺���⿼�����ӷ���ʽ����д��ʵ��ԭ���ķ����ͳ����ܽ�ƽ����ؼ��㡣

 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�