��Ŀ����
���н���ʵ������ķ�Ӧ����ʽ����ȷ����
- A.����Al����NaOH ��Һ�в����������ݣ�2Al+2OH-+2H2O=2AlO2-+3H2��
- B.��ʢ�ж�������������Թܵ�����ˮ�У������Ϊ��ɫ����Һ������Թܣ�3NO2+H2O=2HNO3+NO
- C.����KI��Һ��H2SO4�ữ��H2O2��Һ��ϣ���Һ������2I-+H2O2+2H+=2H2O+I2
- D.�������ʵ���֮��Ϊ1��2��������Һ��Ba��OH��2��Һ��ϣ����ɰ�ɫ������Al3++2SO42-+2Ba2++3OH-=2BaSO4��+Al��OH��3��
������A����������ǿ����Һ��Ӧ����ƫ�����κ�������
B������������ˮ��Ӧ�����������ɫ����һ��������
C��˫��ˮ���������ԣ��ܹ��������������ɵⵥ�ʣ�
D��������Һ�������Ӻ����������ӵ����ʵ���֮����1��4��������ƫ��������ӣ�û�����������������ɣ�
���A����������������Һ��Ӧ�Ļ�ѧ����ʽ�ǣ�2Al+2OH-+2H2O=2AlO2-+3H2������A����
B������������ˮ��Ӧ�Ļ�ѧ����ʽ�ǣ�3NO2+H2O=2HNO3+NO����B����
C��˫��ˮ�ܹ����������ӣ���Ӧ�Ļ�ѧ����ʽ�ǣ�2I-+H2O2+2H+=2H2O+I2����C����
D���������ʵ���֮��Ϊ1��2��������Һ��Ba��OH��2��Һ��ϣ���Һ�������Ӻ����������ӵ����ʵ�����1��4��������ƫ��������ӣ���ȷ���ӷ���ʽ�ǣ�Al3++2SO42-+2Ba2++4OH-=2BaSO4��+AlO2-��+2H2O����D��ȷ��
��ѡD��
���������⿼���˻�ѧ����ʽ����д��ע��D�������Ӻ����������ӵ����ʵ����Ĺ�ϵ�������ѶȲ���

| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | --- |
��2�����һ����ʵ�鷽����֤���������õĿ�״�������к��н���������ʵ�������Լ���
��3��ʵ�����ܽ������������Լ�����õ���
A��Ũ���� B��ϡ���� ������C��ϡ���� ������D������������Һ
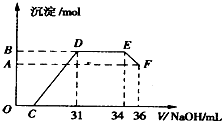
��ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�ͣ�Ϊ�˲ⶨ�����������ֽ��������ʵ���֮�ȣ�ijͬѧȡһ������������������������ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���4mol/L������������Һ����������������Һ�������mL��������ij��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ���Իش��������⣺
��1����μ���4mol/L������������Һ�Ķ�����������
��2��������ͼ�η�����֪����Һ�н��OH-������ǿ��������
��3��ͨ����ͼ�����Լ��㣬�����������ֽ��������ʵ���֮��n��Fe����n��Al��=
 ij�о���ѧϰС������ȷ�Ӧʵ��չ���о������и��л�ѧ�̲��жԡ����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â������ֽ©�����²����մ���������������ɳ�С������ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�
ij�о���ѧϰС������ȷ�Ӧʵ��չ���о������и��л�ѧ�̲��жԡ����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â������ֽ©�����²����մ���������������ɳ�С������ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | -- |
��2�����һ����ʵ�鷽����֤���������õĿ�״�������к��н���������ʵ�������Լ���
��3��ʵ�����ܽ������������Լ�����õ���
A��Ũ���� B��ϡ���� C��ϡ���� D������������Һ
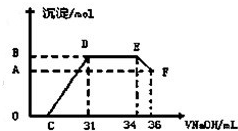
��ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�ͣ�ijͬѧȡһ������������������һ������ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���4mol?L-1������������Һ����������������Һ�������mL��������ij��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ����B���Ӧ�ij��������ʵ���Ϊ
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | -- |
�������������������
��2�����һ����ʵ�鷽����֤���������õĿ�״�������к��н���������ʵ�������Լ���
��Ӧ�����ӷ���ʽΪ
��3��ʵ�����ܽ������������Լ�����õ���
A��Ũ���� B��ϡ���� C��ϡ���� D������������Һ
��ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�ͣ�ijͬѧȡһ������������������һ������ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���4mol?L-1������������Һ����������������Һ�������mL��������ij��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ���Իش��������⣺
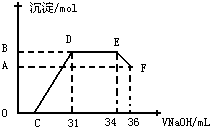
��1��ͼ��OC��û�г������ɣ��˽η�����Ӧ�����ӷ���ʽΪ
��2����DE�Σ����������ʵ���û�б仯����˽η�����Ӧ�����ӷ���ʽΪ
��3��B���Ӧ�ij��������ʵ���Ϊ
�� �� | Al | Al2O3 | Fe | Fe2O3 |
�۵�/�� | 660 | 2 054 | 1 535 | 1 462 |
�е�/�� | 2 467 | 2 980 | 2 750 | �� |
I����1��ijͬѧ�Ʋ⣬���ȷ�Ӧ���õ���������Ӧ�������Ͻ������ǣ��÷�Ӧ�ų�������ʹ���ۻ����������۵�����ͣ���ʱҺ̬���������ۺ��γ������Ͻ�����Ϊ���Ľ����Ƿ��������___________���������������������
��2�����һ����ʵ�鷽����֤���������õĿ�״���������Ƿ��н���������ʵ�������Լ���_____________�����ܷ�����Ӧ�����ӷ���ʽΪ______________________________��
��3��ʵ�����ܽ������������Լ�����õ���___________������ţ���
A.Ũ���� B.ϡ���� C.ϡ���� D.����������Һ
��.ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�͡�ijͬѧȡһ������������������һ������ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���4 mol��L��1������������Һ����������������Һ����� (mL)������ij��������ʵ���(mol)�Ĺ�ϵ����ͼ��ʾ���Իش��������⣺
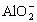
��4��ͼ��OC��û�г������ɣ��˽η�����Ӧ�����ӷ���ʽΪ______________________��
��5����DE�Σ����������ʵ���û�б仯����˽η�����Ӧ�����ӷ���ʽΪ_____________����������˵����Һ��____________���OH����������__________ǿ�������ӷ��ţ���
��6��B��A�IJ�ֵΪ_________mol��
��7��B���Ӧ�ij��������ʵ���Ϊ____________mol��C���Ӧ������������Һ�����Ϊ___________mL��
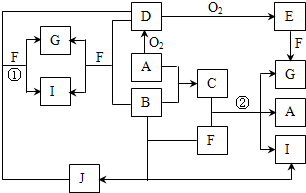 �ɶ�����Ԫ����ɵ�10������A��J֮������ͼ��ϵ����֪��A��BΪͬ��������Ԫ�صĵ��ʣ������Ϊ�����ͨ�������AΪ���壬B��DΪ������B�ʻ���ɫ��FΪҺ�壬A��G��Ũ��Һ����ʱ��Ӧ����D��F��J�ڹ���ʱ��I���ɣ�
�ɶ�����Ԫ����ɵ�10������A��J֮������ͼ��ϵ����֪��A��BΪͬ��������Ԫ�صĵ��ʣ������Ϊ�����ͨ�������AΪ���壬B��DΪ������B�ʻ���ɫ��FΪҺ�壬A��G��Ũ��Һ����ʱ��Ӧ����D��F��J�ڹ���ʱ��I���ɣ�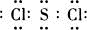
 MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O