��Ŀ����
ij�о���ѧϰС������ȷ�Ӧʵ��չ���о������и��л�ѧ�̲�(����ѡ��)�жԡ����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â����ֽ©�����²����մ���������������ɳ�С������ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£��� �� | Al | Al2O3 | Fe | Fe2O3 |
�۵�/�� | 660 | 2 054 | 1 535 | 1 462 |
�е�/�� | 2 467 | 2 980 | 2 750 | �� |
I����1��ijͬѧ�Ʋ⣬���ȷ�Ӧ���õ���������Ӧ�������Ͻ������ǣ��÷�Ӧ�ų�������ʹ���ۻ����������۵�����ͣ���ʱҺ̬���������ۺ��γ������Ͻ�����Ϊ���Ľ����Ƿ��������___________���������������������
��2�����һ����ʵ�鷽����֤���������õĿ�״���������Ƿ��н���������ʵ�������Լ���_____________�����ܷ�����Ӧ�����ӷ���ʽΪ______________________________��
��3��ʵ�����ܽ������������Լ�����õ���___________������ţ���
A.Ũ���� B.ϡ���� C.ϡ���� D.����������Һ
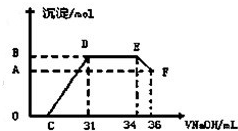
��.ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�͡�ijͬѧȡһ������������������һ������ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���4 mol��L��1������������Һ����������������Һ����� (mL)������ij��������ʵ���(mol)�Ĺ�ϵ����ͼ��ʾ���Իش��������⣺
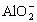
��4��ͼ��OC��û�г������ɣ��˽η�����Ӧ�����ӷ���ʽΪ______________________��
��5����DE�Σ����������ʵ���û�б仯����˽η�����Ӧ�����ӷ���ʽΪ_____________����������˵����Һ��____________���OH����������__________ǿ�������ӷ��ţ���
��6��B��A�IJ�ֵΪ_________mol��
��7��B���Ӧ�ij��������ʵ���Ϊ____________mol��C���Ӧ������������Һ�����Ϊ___________mL��
��.(1)����
(2)NaOH��Һ 2Al+2OH��+2H2O====![]() +3H2��
+3H2��
(3)B
��.(4)H+ + OH�� ==== H2O
(5)![]() + OH��
+ OH�� ![]() NH3��H2O Al3����Fe3����H��
NH3��H2O Al3����Fe3����H�� ![]()
(6)0.008
(7)0.0327
��������1�����ȷ�Ӧ�������¶����Խ��������ۻ������Ӧ���γ������Ͻ𡣣�2������֪�����Ŀ�״���������NaOH��Һ�У�һ��ʱ���ȡ������ϴ�ӡ�������������ɸ��������Ƿ��б仯�ó��Ƿ�Ϊ�����Ͻ�Ľ��ۡ����ܷ�����Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O====![]() +3H2������3����״��������FeΪ����Ӧѡ�������ܽ�Fe�Ҳ������ж������ϡH2SO4��
+3H2������3����״��������FeΪ����Ӧѡ�������ܽ�Fe�Ҳ������ж������ϡH2SO4��
EF�Σ�
Al(OH)3 + OH-====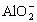 +2H2O��Al
+2H2O��Al
0.008 mol (36-34)��10-3��4 mol 0.008 mol
B��A�IJ�ֵ��Al(OH)3�����ʵ�����ȣ�Ϊ0.008 mol��
DE�Σ�
![]() + OH-
+ OH- ![]() NH3��H2O
NH3��H2O
0.012 mol (34-31)��10-3��4 mol
��������HNO3�����ã�
![]()
![]()
![]() ��Al
��Al![]() Al3+��Fe
Al3+��Fe![]() Fe3+
Fe3+
�ݵ��ӵ�ʧ�غ��У�
0.012 mol��8=0.008 mol��3+n(Fe)��3
�ã�n��Fe(OH)3��=n(Fe)=0.024 mol
B��Fe(OH)3��Al(OH3)����
0.008 mol+0.024 mol=0.032 mol
�����31 mL�������Ƶ���Һ����NaOH:
31��10
CD��Fe3+��Al3+����NaOH:
3��0.008 mol+3��0.024 mol=0.096 mol
OC��H+����NaOH:
0.124 mol-0.096 mol=0.028 mol
C�����NaOH��Һ�����:
![]() =
=

 ij�о���ѧϰС������ȷ�Ӧʵ��չ���о������и��л�ѧ�̲��жԡ����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â������ֽ©�����²����մ���������������ɳ�С������ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�
ij�о���ѧϰС������ȷ�Ӧʵ��չ���о������и��л�ѧ�̲��жԡ����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â������ֽ©�����²����մ���������������ɳ�С������ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | -- |
��2�����һ����ʵ�鷽����֤���������õĿ�״�������к��н���������ʵ�������Լ���
��3��ʵ�����ܽ������������Լ�����õ���
A��Ũ���� B��ϡ���� C��ϡ���� D������������Һ
��ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�ͣ�ijͬѧȡһ������������������һ������ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���4mol?L-1������������Һ����������������Һ�������mL��������ij��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ����B���Ӧ�ij��������ʵ���Ϊ
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | -- |
�������������������
��2�����һ����ʵ�鷽����֤���������õĿ�״�������к��н���������ʵ�������Լ���
��Ӧ�����ӷ���ʽΪ
��3��ʵ�����ܽ������������Լ�����õ���
A��Ũ���� B��ϡ���� C��ϡ���� D������������Һ
��ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�ͣ�ijͬѧȡһ������������������һ������ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���4mol?L-1������������Һ����������������Һ�������mL��������ij��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ���Իش��������⣺
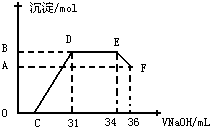
��1��ͼ��OC��û�г������ɣ��˽η�����Ӧ�����ӷ���ʽΪ
��2����DE�Σ����������ʵ���û�б仯����˽η�����Ӧ�����ӷ���ʽΪ
��3��B���Ӧ�ij��������ʵ���Ϊ

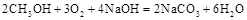 ��
��
 ���� ���a����b�����缫��
���� ���a����b�����缫�� ��3���ҳ��з�����Ӧ�����ӷ���ʽΪ ��
��3���ҳ��з�����Ӧ�����ӷ���ʽΪ ��
 (��״����)����μ���A���IJ��� ��
(��״����)����μ���A���IJ��� ��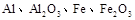 �۵㡢�е��������£�
�۵㡢�е��������£�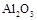
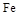
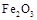
 62
62 750
750 ��
�� �Լ��������˵��Լ���
�Լ��������˵��Լ���