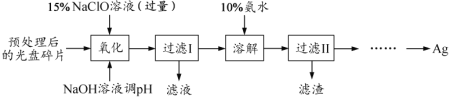
��Ŀ����
����Ŀ����NAΪ�����ӵ�������ֵ������������ȷ����(����)
A. 100 mL 0.1 mol��L��1 K2SO4��Һ�У�������ԭ����Ϊ0.04NA
B. 1 mol Na������O2��Ӧ������Na2O��Na2O2�Ļ���ת�Ƶĵ�����ΪNA
C. 25 ��ʱ��1.0 L pH��13��Ba(OH)2��Һ�У����е�OH����ĿΪ0.2NA
D. 100 g 17%�İ�ˮ�У����а�������ΪNA
���𰸡�B
��������A�100mL 0.1molL-1 K2SO4��Һ��SO42-�к�����ԭ��=0.1 L��0.1molL-1��4=0.04mol����ˮ�л��кܶ���ԭ�ӣ���A����B�1 mol Na������O2��Ӧ����������Na2O����Na2O2��Na������0�۱�Ϊ+1�ۣ�����ת�Ƶ�����ΪNA����B��ȷ��C�25 ��ʱ��1.0 L pH��13��Ba(OH)2��Һ����n(OH-)=1.0 L��![]() mol/L=0.1mol�����Ժ��е�OH-��ĿΪ0.1NA����C����D�100 g 17%�İ�ˮ�У���Ҫ������ʽ��һˮ�ϰ������еİ���������С��NA����D����
mol/L=0.1mol�����Ժ��е�OH-��ĿΪ0.1NA����C����D�100 g 17%�İ�ˮ�У���Ҫ������ʽ��һˮ�ϰ������еİ���������С��NA����D����

��ϰ��ϵ�д�
 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�����Ŀ