��Ŀ����
����Ŀ�����ĵ��ʡ��Ͻ��仯������;�dz��㷺��
(1)Ni2����̬��������Ų�ʽΪ________��
(2)��״���ɴ�CH2=CHC��N��������CH3CH2C��N��CH2=CHC��N��������������������Ŀ��n(��)��n(��)=________��CH3CH2C��N������̼ԭ�ӹ�����ӻ�����Ϊ________��
(3)[Ni(N2H4)2](N3)2��һ�ָ������ܲ��ϡ�����N2H4����ˮ���ܣ�����Ϊ���Ƕ��Ǽ��Է����⣬����Ϊ______________________��[Ni(N2H4)2]2���к��ĸ���λ���������ǿռ乹�ͣ�[Ni(N2H4)2]2���Ľṹ����ʾ��ͼ��ʾΪ______________________��
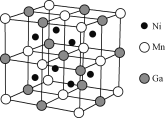
(4)һ�����͵Ĺ��ܲ��ϵľ����ṹ����ͼ��ʾ�����Ļ�ѧʽ�ɱ�ʾΪ________��
���𰸡�[Ar]3d8��1s22s22p63s23p63d8 2��1 sp��sp3 N2H4��H2O֮������γ���� ![]() Ni2MnGa
Ni2MnGa
��������
(1)Ni��28��Ԫ�أ�ԭ�Ӻ�������Ų�ʽ��1s22s22p63s23p63d84s2��Niԭ��ʧȥ4s�ܼ�2�������γ�Ni2+��
(2)CH2=CHCN�к���3��C-H����1��C-C������1��C=C˫����1��C��N����������Ϊ������˫������1��������1����������������1��������2��������������̼ԭ�Ӿ��γ�4��������û�й¶Ե��ӣ��ӻ������ĿΪ4����-CN��̼ԭ���γ�2��������Ҳû�й¶Ե��ӣ��ӻ������ĿΪ2��
(3)����N2H4��H2O֮������γ������Ni2+�ṩ�չ����N2H4��Nԭ���ṩ�¶Ե��ӣ��γ���λ����
(4)��̯�����㾧����Ni��Ga��Mnԭ����Ŀ��ȷ����ѧʽ��
(1)Ni��28��Ԫ�أ�ԭ�Ӻ�������Ų�ʽ��1s22s22p63s23p63d84s2��Niԭ��ʧȥ4s�ܼ�2�������γ�Ni2+����Ni2+��̬��������Ų�ʽΪ1s22s22p63s23p63d8��
(2)CH2=CHCN�к���3��C-H����1��C-C������1��C=C˫����1��C��N����������Ϊ������˫������1��������1����������������1��������2�����������������������������Ŀ��n(��)��n(��)=(3+1+1+1)��(1+2)=2��1��������̼ԭ�Ӿ��γ�4��������û�й¶Ե��ӣ��ӻ������ĿΪ4��̼ԭ�Ӳ�ȡsp3�ӻ�����-CN��̼ԭ���γ�2��������Ҳû�й¶Ե��ӣ��ӻ������ĿΪ2��̼ԭ�Ӳ�ȡsp�ӻ���
(3)����N2H4��H2O֮������γ������ʹ����N2H4����ˮ���ܣ�Ni2+�ṩ�չ����N2H4��Nԭ���ṩ�¶Ե��ӣ��γ���λ����[Ni(N2H4)2]2+�Ľṹ����ʾ��ͼ��ʾΪ��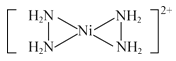 ��
��
(4)Niԭ�Ӵ���С���������ģ�������Niԭ����Ŀ1��8=8��Mnԭ�Ӵ��ڶ��������ģ�������Mnԭ����Ŀ=8��![]() +6��
+6��![]() =4��Ga���ھ������������ģ�������Gaԭ����Ŀ=1+12��
=4��Ga���ھ������������ģ�������Gaԭ����Ŀ=1+12��![]() =4������Ni��Mn��Gaԭ����Ŀ֮��=8��4��4=2��1��1���ʾ���Ļ�ѧʽΪ��Ni2MnGa��
=4������Ni��Mn��Gaԭ����Ŀ֮��=8��4��4=2��1��1���ʾ���Ļ�ѧʽΪ��Ni2MnGa��

 �������ϵ�д�
�������ϵ�д�����Ŀ����֪��һԪ����HA�ĵ���ƽ�ⳣ��K ��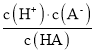 ��25��ʱ��CH3COOH��HCN��H2CO3�ĵ���ƽ�ⳣ�����£�
��25��ʱ��CH3COOH��HCN��H2CO3�ĵ���ƽ�ⳣ�����£�
��ѧʽ | CH3COOH | HCN | H2CO3 |
K | 1.75��10�C5 | 4.9��10�C10 | K1 = 4.4��10�C7 K2 = 5.6��10�C11 |
����˵����ȷ����
A. ϡ��CH3COOH��Һ�Ĺ����У�n(CH3COO�C)��С
B. NaHCO3��Һ�У�c(H2CO3) �� c(![]() ) �� c(HCO3)
) �� c(HCO3)
C. 25��ʱ����ͬ���ʵ���Ũ�ȵ�NaCN��Һ�ļ���ǿ��CH3COONa��Һ
D. ��CH3COOH��Һ��HCN��Һ�м���Na2CO3��Һ��������CO2