��Ŀ����
ijͬѧ��������ʵ��������ش�������⣺��1��ȡ30mL��ˮ��������ȵ�T��ʱ�����е�c��H+��=2.0��10-7mol?L-1����c��OH-��=
��2����ȴ�ָ������º���ˮ�е���ϡ����ʹ��Һ���Ϊ100mL����ʱ��Һ�е�c��H+��=5.0��10-4mol?L-1�����ʱ��Һ�е�c��OH-��Ϊ
��3��ȡ��2������Һ20mL�������мӰ�ˮ����ǡ�÷�Ӧ��ȫ����������Һ������Ũ�ȵĴ�С��ϵΪ
��4��ȡ��2������Һ50mL���ö��Ե缫���е�⣬�������ĵ缫��ӦΪ
��5������2����ʣ����Һ����5.0��10-4 mol?L-1��NaOH��Һ�кͣ���ҪNaOH��Һ�����Ϊ
��������1����ˮ�������������Ũ�ȵ�����ˮ����������������ӵ�Ũ�ȣ�
��2��������Kw=c��H+��?c��OH-��=10-14����c��OH-��=
��
��3��ǡ����ȫ��Ӧ�õ��������Һ������笠�����ˮ�������������Ũ�ȴ�С��
��4����������������ʧȥ��������������ˮ������Ϊ�����ӵõ����������������൱�ڵ��ˮ��
��5�������кͷ�Ӧ����ʽ������������ӵ����ʵ�����Ȼ��������ʵ���Ũ�ȹ�ʽ���������
��2��������Kw=c��H+��?c��OH-��=10-14����c��OH-��=
| Kw |
| c(H+) |
��3��ǡ����ȫ��Ӧ�õ��������Һ������笠�����ˮ�������������Ũ�ȴ�С��
��4����������������ʧȥ��������������ˮ������Ϊ�����ӵõ����������������൱�ڵ��ˮ��
��5�������кͷ�Ӧ����ʽ������������ӵ����ʵ�����Ȼ��������ʵ���Ũ�ȹ�ʽ���������
����⣺��1����ˮ�������������Ũ�ȵ�����ˮ����������������ӵ�Ũ�ȣ�c��OH-��=c��H+��=2.0��10-7mol?L-1���ʴ�Ϊ��2.0��10-7��
��2��������Kw=c��H+��?c��OH-��=10-14����c��OH-��=
=
mol?L-1=2��10-11mol?L-1���ʴ�Ϊ��2��10-11��
��3��ǡ����ȫ��Ӧ�õ��������Һ��笠�����ˮ�����������c��NH4+����c��SO42-����笠�����ˮ����Һ�����ԣ�����c��H+����c��OH-�����������Ũ�ȵĴ�С��ϵΪc��NH4+����c��SO42-����c��H+����c��OH-����
�ʴ�Ϊ��c��NH4+����c��SO42-����c��H+����c��OH-����
��4����������������ʧȥ��������������ˮ�������缫��Ӧʽ��4OH--4e-=O2��+2H2O������Ϊ�����ӵõ����������������൱�ڵ��ˮ�����ϡ������Һ������Ũ������pHֵ��С��
�ʴ�Ϊ��4OH--4e-=O2��+2H2O����С��
��5��ϡ������Һ��n��H+��=0.1L��5.0��10-4mol?L-1=5��10-5 mol������H++OH-=H2O��֪��n��NaOH��=c��OH-��=n��H+��=5��10-5 mol������ҪNaOH��Һ�����V=
=0.1L����100ml���ʴ�Ϊ��100��
��2��������Kw=c��H+��?c��OH-��=10-14����c��OH-��=
| Kw |
| c(H+) |
| 10-14 |
| 5.0��10-4 |
��3��ǡ����ȫ��Ӧ�õ��������Һ��笠�����ˮ�����������c��NH4+����c��SO42-����笠�����ˮ����Һ�����ԣ�����c��H+����c��OH-�����������Ũ�ȵĴ�С��ϵΪc��NH4+����c��SO42-����c��H+����c��OH-����
�ʴ�Ϊ��c��NH4+����c��SO42-����c��H+����c��OH-����
��4����������������ʧȥ��������������ˮ�������缫��Ӧʽ��4OH--4e-=O2��+2H2O������Ϊ�����ӵõ����������������൱�ڵ��ˮ�����ϡ������Һ������Ũ������pHֵ��С��
�ʴ�Ϊ��4OH--4e-=O2��+2H2O����С��
��5��ϡ������Һ��n��H+��=0.1L��5.0��10-4mol?L-1=5��10-5 mol������H++OH-=H2O��֪��n��NaOH��=c��OH-��=n��H+��=5��10-5 mol������ҪNaOH��Һ�����V=
| 5��10-5 mol |
| 5.0��10-4 mol?L -1 |
���������⿼��֪ʶ���Ϊȫ�棬�漰˯�ĵ��롢���ӻ�����������ˮ�⡢����ԭ����֪ʶ����Ŀ�Ѷ��еȣ�����ˮ����ʵĵ���ƽ�⡢笠����ӵ�ˮ��ƽ�⡢���ԭ���ǽ���Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
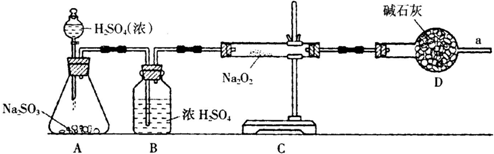


 H+ +ClO-��Ϊ��̽��HClO��Ư���ԣ�ij��ѧ��ȤС���A��B��C��D��ѡ�������Ʊ���������������������������ʵ�顣
H+ +ClO-��Ϊ��̽��HClO��Ư���ԣ�ij��ѧ��ȤС���A��B��C��D��ѡ�������Ʊ���������������������������ʵ�顣 
