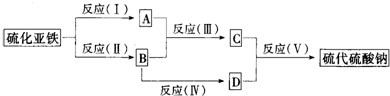
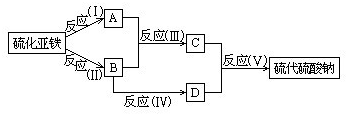
��Ŀ����
����A��B��C��D����ԭ������������������ֶ�����Ԫ�أ���������������������C��ԭ��������A��B��ԭ������֮�ͣ�A��C��D��������������Ϊ13��
��D��ԭ��������C��2����D������������Ӧ��ˮ�����Ƕ�Ԫǿ�ᣮ
�Ը������������ش�
��1��B���ʵĵ���ʽΪ ������CԪ�ص�ԭ�ӽṹʾ��ͼ ��
��2�����л���������B��C�γɵĻ������йص��� ��
A������ЧӦ B���⻯ѧ���� C������ D��PM2.5
��3��A��B��C��D����Ԫ�ؿ��γ�һ�������ԭ�Ӹ���֮��Ϊ8��2��4��1���û��������� ��������ͣ���
�����������ж�����Ԫ��������ʵ����ݣ�
| Ԫ�ر�� Ԫ������ | �� | �� | �� | �� | �� | �� | �� |
| ԭ�Ӱ뾶��10-10m�� | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 |
| ��������ϼ� | +2 | +1 | +5 | +7 | +1 | +5 | |
| ������ϼ� | -2 | -3 | -1 | -3 |
��1��Ԫ�آ������ڱ��е�λ�� ��Ԫ�آڵĵ�����CO2��Ӧ�Ļ�ѧ����ʽ ���õ��ʿ�ͨ����ⷨ�Ƶã����н������������翪�������õ��� ��
A���� B���� C���� D��ͭ
��2��Ԫ�آ���Ԫ�آ���Ƚϣ���̬�⻯����ȶ����� ����ṹʽ����
��3��Ԫ�آ��γɵ�+3��+5�۵��Ȼ����У���ԭ�Ӿ��ﵽ8�����ȶ��ṹ�Ļ������� ����д��ѧʽ��
���𰸡�����������A��B��C��D����ԭ������������������ֶ�����Ԫ�أ�D������������Ӧ��ˮ�����Ƕ�Ԫǿ�ᣬ��DΪ��Ԫ�أ�D��ԭ��������C��2������C��ԭ������Ϊ8����CΪ��Ԫ�أ�A��C��D��������������Ϊ13����A������������Ϊ13-6-6=1��A���ڢ�A�壬��AΪH����B��ԭ������Ϊ8-1=7����BΪ��Ԫ�أ���AΪLi����B��ԭ������Ϊ8-3=5����BΪ��Ԫ�أ�����⣨2����֪��AΪ��Ԫ�ء�BΪ��Ԫ�أ��ݴ˽��
����ֻ��-2�ۣ�û�������ϼۣ���ΪOԪ�أ��ۢ���+1�ۣ����ڢ�A�壬ԭ�Ӱ뾶�ۣ��ޣ��Ң۵�ԭ�Ӱ뾶������С���ʢ�ΪLiԪ�ء���ΪNaԪ�أ���ֻ��+2�ۣ����ڢ�A�壬ԭ�Ӱ뾶����Liԭ�ӣ��ʢ�ΪMgԪ�أ��ܢ߶���+5��-3�ۣ����ڢ�A�壬ԭ�Ӱ뾶�ܣ��ߣ��ʢ�ΪPԪ�ء���ΪNԪ�أ�����+7��-1�ۣ���ΪClԪ�أ��ݴ˽��
����⣺����A��B��C��D����ԭ������������������ֶ�����Ԫ�أ�D������������Ӧ��ˮ�����Ƕ�Ԫǿ�ᣬ��DΪ��Ԫ�أ�D��ԭ��������C��2������C��ԭ������Ϊ8����CΪ��Ԫ�أ�A��C��D��������������Ϊ13����A������������Ϊ13-6-6=1��A���ڢ�A�壬��AΪH����B��ԭ������Ϊ8-1=7����BΪ��Ԫ�أ���AΪLi����B��ԭ������Ϊ8-3=5����BΪ��Ԫ�أ�����⣨2����֪��AΪ��Ԫ�ء�BΪ��Ԫ�أ�
��1��B����Ϊ�����������е�ԭ��֮���γ�3�Թ��õ��Ӷԣ��ʵ���ʽΪΪ ��
��
OԪ��������Ϊ8�����������Ϊ8����2�����Ӳ㣬����������Ϊ6������ԭ�ӽṹʾ��ͼ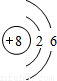 ��
��
�ʴ�Ϊ��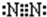 ��
��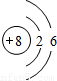 ��
��
��2����Ԫ�ص���������Ե��¹⻯ѧ���������꣬PM2.5���ɷ۳�����������ЧӦ�ɶ�����̼�ȵ��£�
�ʴ�Ϊ��BC��
��3��A��B��C��D����Ԫ�ؿ��γ�һ�������ԭ�Ӹ���֮��Ϊ8��2��4��1���û������ǣ�NH4��2SO4���������Ӿ��壬
�ʴ�Ϊ�����Ӿ��壻
����ֻ��-2�ۣ�û�������ϼۣ���ΪOԪ�أ��ۢ���+1�ۣ����ڢ�A�壬ԭ�Ӱ뾶�ۣ��ޣ��Ң۵�ԭ�Ӱ뾶������С���ʢ�ΪLiԪ�ء���ΪNaԪ�أ���ֻ��+2�ۣ����ڢ�A�壬ԭ�Ӱ뾶����Liԭ�ӣ��ʢ�ΪMgԪ�أ��ܢ߶���+5��-3�ۣ����ڢ�A�壬ԭ�Ӱ뾶�ܣ��ߣ��ʢ�ΪPԪ�ء���ΪNԪ�أ�����+7��-1�ۣ���ΪClԪ�أ�
��1��Ԫ�آ���ClԪ�أ������ڱ��е�λ���ǵ������ڵڢ�A�壻
Ԫ�آ���Mg��Mg������CO2��ȼ������̼������þ����Ӧ�Ļ�ѧ����ʽΪ��2Mg+CO2 2MgO+C��
2MgO+C��
�������翪�������õĽ����������ϴ���Բ����ã������翪�����õĽ�������Cu��
�ʴ�Ϊ���������ڵڢ�A�壻2Mg+CO2 2MgO+C��D��
2MgO+C��D��
��2���ǽ�����N��P����NH3���ȶ�����ṹʽΪ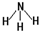 ��
��
�ʴ�Ϊ��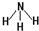 ��
��
��3��Ԫ�آ�ΪPԪ�أ��γɵ�+3��+5�۵��Ȼ����У�PCl3�и�ԭ�Ӿ��ﵽ8�����ȶ��ṹ��
�ʴ�Ϊ��PCl3��
���������⿼��ṹ����λ�ù�ϵ�����û�ѧ���Ԫ�ػ���������ʵȣ������и��ݻ��ϼ���ԭ�Ӱ뾶�ƶ�Ԫ���ǽ���ؼ���ע�����֪ʶ���������գ�
����ֻ��-2�ۣ�û�������ϼۣ���ΪOԪ�أ��ۢ���+1�ۣ����ڢ�A�壬ԭ�Ӱ뾶�ۣ��ޣ��Ң۵�ԭ�Ӱ뾶������С���ʢ�ΪLiԪ�ء���ΪNaԪ�أ���ֻ��+2�ۣ����ڢ�A�壬ԭ�Ӱ뾶����Liԭ�ӣ��ʢ�ΪMgԪ�أ��ܢ߶���+5��-3�ۣ����ڢ�A�壬ԭ�Ӱ뾶�ܣ��ߣ��ʢ�ΪPԪ�ء���ΪNԪ�أ�����+7��-1�ۣ���ΪClԪ�أ��ݴ˽��
����⣺����A��B��C��D����ԭ������������������ֶ�����Ԫ�أ�D������������Ӧ��ˮ�����Ƕ�Ԫǿ�ᣬ��DΪ��Ԫ�أ�D��ԭ��������C��2������C��ԭ������Ϊ8����CΪ��Ԫ�أ�A��C��D��������������Ϊ13����A������������Ϊ13-6-6=1��A���ڢ�A�壬��AΪH����B��ԭ������Ϊ8-1=7����BΪ��Ԫ�أ���AΪLi����B��ԭ������Ϊ8-3=5����BΪ��Ԫ�أ�����⣨2����֪��AΪ��Ԫ�ء�BΪ��Ԫ�أ�
��1��B����Ϊ�����������е�ԭ��֮���γ�3�Թ��õ��Ӷԣ��ʵ���ʽΪΪ
 ��
��OԪ��������Ϊ8�����������Ϊ8����2�����Ӳ㣬����������Ϊ6������ԭ�ӽṹʾ��ͼ
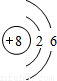 ��
���ʴ�Ϊ��
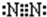 ��
��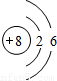 ��
����2����Ԫ�ص���������Ե��¹⻯ѧ���������꣬PM2.5���ɷ۳�����������ЧӦ�ɶ�����̼�ȵ��£�
�ʴ�Ϊ��BC��
��3��A��B��C��D����Ԫ�ؿ��γ�һ�������ԭ�Ӹ���֮��Ϊ8��2��4��1���û������ǣ�NH4��2SO4���������Ӿ��壬
�ʴ�Ϊ�����Ӿ��壻
����ֻ��-2�ۣ�û�������ϼۣ���ΪOԪ�أ��ۢ���+1�ۣ����ڢ�A�壬ԭ�Ӱ뾶�ۣ��ޣ��Ң۵�ԭ�Ӱ뾶������С���ʢ�ΪLiԪ�ء���ΪNaԪ�أ���ֻ��+2�ۣ����ڢ�A�壬ԭ�Ӱ뾶����Liԭ�ӣ��ʢ�ΪMgԪ�أ��ܢ߶���+5��-3�ۣ����ڢ�A�壬ԭ�Ӱ뾶�ܣ��ߣ��ʢ�ΪPԪ�ء���ΪNԪ�أ�����+7��-1�ۣ���ΪClԪ�أ�
��1��Ԫ�آ���ClԪ�أ������ڱ��е�λ���ǵ������ڵڢ�A�壻
Ԫ�آ���Mg��Mg������CO2��ȼ������̼������þ����Ӧ�Ļ�ѧ����ʽΪ��2Mg+CO2
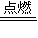 2MgO+C��
2MgO+C���������翪�������õĽ����������ϴ���Բ����ã������翪�����õĽ�������Cu��
�ʴ�Ϊ���������ڵڢ�A�壻2Mg+CO2
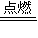 2MgO+C��D��
2MgO+C��D����2���ǽ�����N��P����NH3���ȶ�����ṹʽΪ
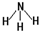 ��
���ʴ�Ϊ��
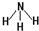 ��
����3��Ԫ�آ�ΪPԪ�أ��γɵ�+3��+5�۵��Ȼ����У�PCl3�и�ԭ�Ӿ��ﵽ8�����ȶ��ṹ��
�ʴ�Ϊ��PCl3��
���������⿼��ṹ����λ�ù�ϵ�����û�ѧ���Ԫ�ػ���������ʵȣ������и��ݻ��ϼ���ԭ�Ӱ뾶�ƶ�Ԫ���ǽ���ؼ���ע�����֪ʶ���������գ�

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ