��Ŀ����
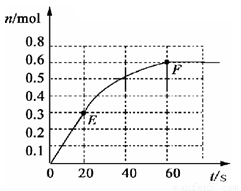
373Kʱ��ij1L�ܱ������з������¿��淴Ӧ��A��g��![]() 22 B��g������������B�����ʵ�����ʱ��仯����ͼ��ʾ������գ�
22 B��g������������B�����ʵ�����ʱ��仯����ͼ��ʾ������գ�
��1����֪373Kʱ60s�ﵽƽ�⣬��ǰ60s��A��ƽ����Ӧ����Ϊ ��
��2����373KʱB��ƽ��Ũ��Ϊ��A��3����473Kʱ�������������䣩��B��ƽ��Ũ��ΪA��2����������ͼ�л���473KʱA�����ʵ�����ʱ��ı仯���ߡ�
��3������Ӧ��373K���У���1L�ܱ������м���1mol B��0.2molHe���ﵽƽ��ʱB��ת�����ǣ�___________������ţ���
A������60�� B������40��
C��С��40�� D������40����60��֮��
��4����֪��������������֮�����ߵ�б�ʱ�ʾ��ʱ����B��ƽ����Ӧ���ʣ�����ֱ��EF��б�ʱ�ʾ20s~60s��B��ƽ����Ӧ���ʣ����Բ�������������һ�������б�ʵ�����
��
��1��0.005mol?L-1?s-1
��2�� ����ͼ������ע��2�㣺A����ʼ��ƽ��ʱ�����ʵ������ﵽƽ������ʱ��ӦС��60s��

��3�� B
��4��ijһʱ�̵ķ�Ӧ���ʣ���ʱ���ʡ�˲ʱ���ʣ�

��ϰ��ϵ�д�
 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�
�����Ŀ
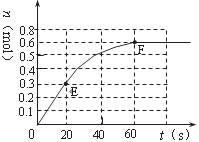
 ��2010?¬������ģ��373Kʱ��ij1L�ܱ������з������¿��淴Ӧ��A��g��?2B��g������������B�����ʵ����仯��ͼ��ʾ��
��2010?¬������ģ��373Kʱ��ij1L�ܱ������з������¿��淴Ӧ��A��g��?2B��g������������B�����ʵ����仯��ͼ��ʾ��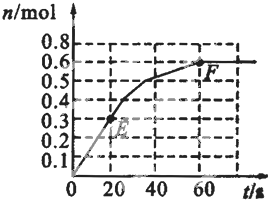 373Kʱ��ij1L�ܱ������з������¿��淴Ӧ��A ��g��?2B ��g������������B�����ʵ����仯��ͼ��ʾ
373Kʱ��ij1L�ܱ������з������¿��淴Ӧ��A ��g��?2B ��g������������B�����ʵ����仯��ͼ��ʾ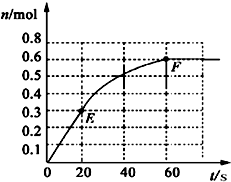 373Kʱ��ij1L�ܱ������м���1molPCl5�������¿��淴Ӧ��
373Kʱ��ij1L�ܱ������м���1molPCl5�������¿��淴Ӧ�� PCl3��g��+Cl2 ��g������������PCl3�����ʵ����仯����ͼ��ʾ��
PCl3��g��+Cl2 ��g������������PCl3�����ʵ����仯����ͼ��ʾ�� N2��g��+ 3 H2��g������������H2�����ʵ����仯����ͼ��ʾ��
N2��g��+ 3 H2��g������������H2�����ʵ����仯����ͼ��ʾ��

 d.�ٳ���N2
d.�ٳ���N2 ��������PCl3�����ʵ����仯����ͼ��ʾ��
��������PCl3�����ʵ����仯����ͼ��ʾ��